Predictive Value of the Standardized Uptake Value of 18F-fluorodeoxyglucose Positron Emission Tomography-Computed Tomography in Assessing Axillary Lymph Node Metastasis in Different Subtypes of Breast Cancer
Article information
Abstract
Purpose
This study aimed to examine the association between the maximum standardized uptake value (SUVmax) of different molecular subtypes of primary breast cancer with axillary lymph node (ALN) metastasis.
Methods
The medical records of 633 patients, who underwent 18F-fluorodeoxyglucose positron emission tomography-computed tomography (PET/CT) for preoperative staging, were retrospectively reviewed. The cohort was stratified by the following molecular subtypes with immunohistochemical examination: luminal A, luminal B, human epidermal growth factor receptor 2 positive (HER2+), and triple negative. We evaluated the optimal cutoff SUVmax to predict ALN metastasis in each subtype using the receiver operating characteristic (ROC) analysis. Moreover, the risk factors for ALN metastasis were evaluated.
Results
Overall, the SUVmax was positively correlated with the number of metastatic ALN (p=0.001). The mean SUVmax was higher in aggressive subtypes (4.5±0.2, 6.1±0.4, 6.5±0.5, and 7.5±0.5 in luminal A, luminal B, HER2+, and triple negative, respectively, p<0.001). Upon ROC analysis, the SUVmax of the HER2+ subtype predicted ALN metastasis most accurately, with a cutoff value of 5.5, area under the curve (AUC) of 0.708, sensitivity of 74.2%, and specificity of 64.6% (p=0.002). The triple negative subtype did not show a significant difference in SUVmax between patients with and without metastasis (p=0.13). Subtype-adjusted SUVmax, HER2 positivity, lymphovascular invasion, and T stage were significant predictors for ALN metastasis.
Conclusion
The SUVmax of primary breast cancer may be an independent predictor of ALN metastasis, being the most accurate in the HER2+ subtype. As PET/CT could facilitate tailored axillary management, this approach could be considered for the initial staging and treatment planning in patients with breast cancer.
INTRODUCTION
Trends in the management of the axilla in patients with early breast cancer have rapidly evolved toward a conservative and less extensive treatment. For the last few decades, axillary lymph node (ALN) dissection had been recommended as the standard procedure in patients with defined ALN metastasis because it was the most accurate method of identification and an important adverse prognostic factor for these patients [1]. However, evidence from recent clinical trials, such as ACOSOG Z0011 and AMAROS, suggested a practice change. As complete ALN dissection did not improve survival in both trials on the basis of effective radiotherapy, de-escalation of axillary surgery is currently allowed in patients with early breast cancer [2,3]. Hence, preoperative prediction of ALN status is becoming increasingly important in the initial treatment planning. Moreover, due to the rapid advancement in the understanding of breast cancer’s molecular biology, we now recognize breast cancer as a heterogeneous disease that needs an individualized treatment approach. Therefore, noninvasive methods providing information on the biologic activity of the tumor and lymph node status preoperatively may be helpful in these patients.
Positron emission tomography-computed tomography (PET/CT) is a widely used imaging tool for evaluating the tumor stage and detecting disease recurrence [4]. PET/CT has advantages over other imaging modalities, as it is capable of showing abnormal metabolic activities prior to the detection of morphological changes in anatomic images. Additionally, PET/CT is useful in predicting lymph node metastasis in patients with cancer [5]. However, in patients with breast cancer, the role of PET/CT in axillary staging has not yet been fully established and has not been recommended for routine examinations [6]. The predictive value of the maximum standardized uptake value (SUVmax) parameter in detecting ALN metastasis among different breast cancer molecular subtypes remains controversial [7,8].
This study aimed to evaluate if the preoperative SUVmax of primary tumor could predict ALN metastasis in patients with different molecular subtypes of breast cancer.
METHODS
Study design and patient selection
A total of 633 patients who underwent 18F-fluorodeoxyglucose (FDG) PET/CT for the initial staging of primary breast cancer between January 2010 and June 2015 were retrospectively enrolled in this study. Patients with carcinoma in situ, de novo stage IV breast cancer, history of neoadjuvant chemotherapy, and diagnosis of N1mi disease or other primary malignancies were excluded. Staging was performed according to the American Joint Committee on Cancer 7th edition guidelines [9]. Data including patient age (<50 or ≥50 years), tumor size (T stage, T1–T4), nodal status (N stage, N0–N3), hormone receptor status, human epidermal growth factor receptor (HER)-2 status, histologic grade (Grades 1, 2, and 3), and lymphovascular invasion (LVI) were obtained from our database, a web-based system that has been collecting information on patients with breast cancer since 1983. The need for informed consent was waived by the Institutional Review Board of the Korean Cancer Center Hospital, which approved the study protocols, due to the retrospective nature of this study (No. 2019-07-012). All procedures performed in studies involving human participants were in accordance with the 1964 Declaration of Helsinki and its later amendments.
Immunohistochemical staining
Formalin-fixed, paraffin-embedded tissue sections were obtained from the surgical specimen and routine immunohistochemical assessment for estrogen receptor (ER), progesterone receptor (PR), HER-2, and Ki67 expression was performed. ER and PR positivity were defined as the expression of the proteins in at least 1% of all tumor cells. HER2 overexpression was defined as a score of 3+ in the immunohistochemical staining or 2+ with positive gene amplification in in situ hybridization. The Scarff-Bloom-Richardson grading system was applied for tumor grading. According to the recommendation of the 12th St Gallen International Breast Cancer Consensus Conference (2011), breast cancers were classified into four molecular subtypes: luminal A (ER- and/or PR-positive, Ki-67<14%, and HER2-negative), luminal B (ER- and/or PR-positive, Ki-67>14%, and HER2-negative or ER and/or PR-positive, any Ki-67 index, and HER2-positive), HER2+ (ER negative, PR-negative, and HER2-positive), and triple negative (ER-negative, PR-negative, and HER2-negative) [10].
FDG PET acquisition
All patients fasted for at least 6 hours prior to 18F-FDG administration (7.4 MBq/kg of body weight, intravenously) and had blood glucose levels <7.2 mmol/L. PET/CT was performed using a Biograph 6 PET/CT scanner (Siemens Medical Solutions, Knoxville, USA). PET/CT images from the vertex to the upper thigh (5–6 bed positions) were acquired 60 minutes after 18F-FDG injection. First, CT images without contrast enhancement were obtained using a 6-slice helical CT scanner (peak voltage of 130 kVp, automated tube current of 30 mA, 0.6 second/CT rotation, and pitch of 6). Then, PET emission data were obtained in the same area for 3.5 minutes per bed position. PET images were reconstructed using a conventional iterative algorithm (ordered subsets expectation-maximization, 2 iterations, and 8 subsets), using CT data for attenuation correction.
Imaging analysis
All PET/CT images were reviewed on an e.soft workstation (Siemens Medical Systems, Iselin, USA). For each patient, a region of interest (ROI) was manually placed on the entire breast lesion using a volumetric analysis template. The SUVmax of the lesion was calculated using decay-corrected activity within the ROI, injected dose, and body weight. All PET/CT images were reviewed and interpreted by three experienced nuclear medicine physicians, and decisions were made based on consensus.
Statistical analysis
We compared discrete variables using the chi-square and Fisher’s exact tests. Student’s t-test on independent variables and analysis of variance were used to compare continuous variables, including mean age and SUVmax between different groups. Results are presented as frequencies and percentages for categorical variables or means±standard deviation for continuous variables. Receiver operating characteristic (ROC) analysis was performed to determine the ideal cutoff SUVmax for detecting ALN metastasis. Univariate and multivariate analyses on the predictors of ALN metastasis detection were performed using logistic regression. Statistical analyses were performed using the SPSS version 25 software (SPSS, Chicago, USA). A p-value<0.05 was considered statistically significant.
RESULTS
Patient characteristics
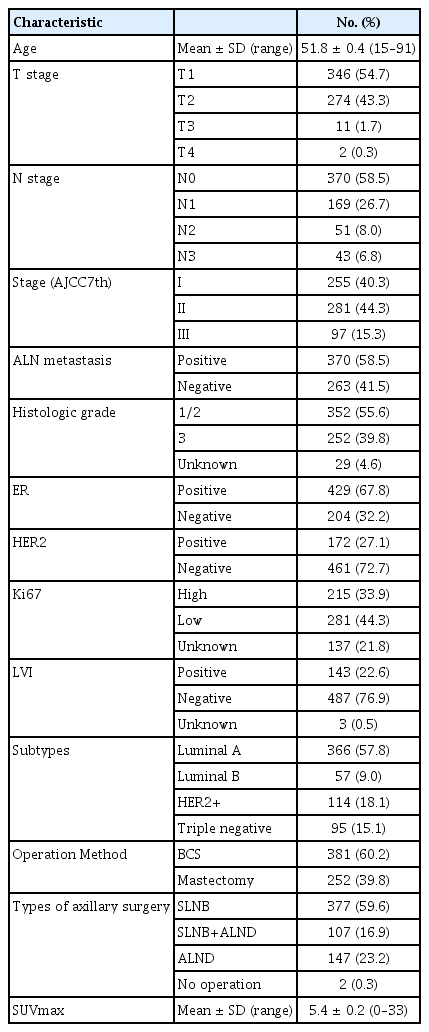
A total of 633 patients with operable, invasive breast cancer were included in the study. Overall, 370 patients (58.5%) were node-positive, and 263 (41.5%) were node-negative. The mean age was 51.8±0.4 years. Tumor staging was as follows: stage I in 255 patients (40.3%), stage II in 281 (44.3%), and stage III in 97 (15.3%). The molecular cancer subtypes were as follows: luminal A in 366 patients (57.8%), luminal B in 57 (9.0%), HER2 positive in 114 (18.1%), and triple negative in 95 (15.1%). The mean SUVmax of the cohort was 5.4±0.2 (Table 1). Clinicopathological findings according to each molecular subtype are presented in Table 2. The percentage of patients with ALN metastasis differed among molecular subtypes (p<0.001).
Association of SUVmax and ALN metastasis
In the entire cohort, the mean SUVmax of patients with and without ALN metastasis were 6.3±3.9 and 4.8±3.9, respectively (Table 3, p<0.001). There was a positive correlation between the increase in SUVmax and number of metastatic ALNs (p<0.001).
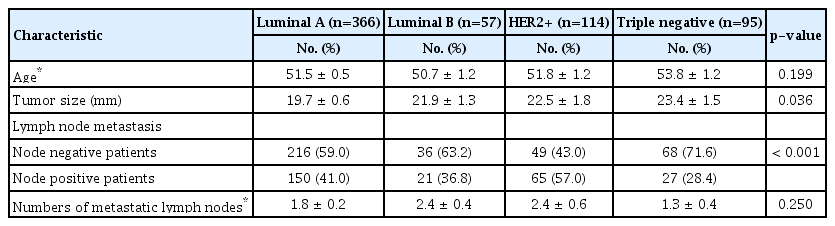
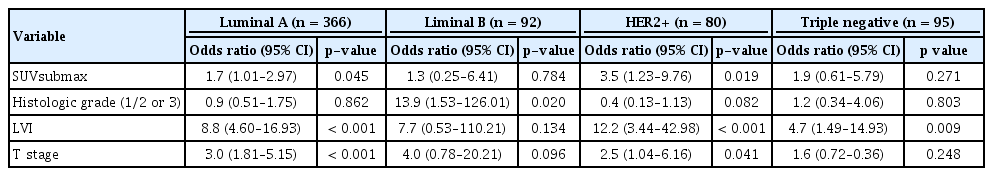
When stratified by subtypes, we found a significant positive correlation between the SUVmax and more biologically aggressive tumors: HER2+ and triple negative subtypes presented higher SUVmax than luminal A and luminal B subtypes (Figure 1). The mean SUVmax were 4.5±0.2, 6.1±0.4, 6.5±0.5, and 7.5±0.5 in luminal A, luminal B, HER2+ and triple negative subtypes, respectively (Table 4, p<0.001). Regarding ALN metastasis, it was associated with the highest frequency to the HER+ subtype (57%), while the lowest frequency was associated with the triple negative subtype (28%, Table 2). Luminal A, luminal B, and HER2+ subtypes showed a statistically significant difference between the primary tumor SUVmax and presence of ALN metastasis. Conversely, we found no significant differences in the triple negative subtype (Table 4, p=0.130).
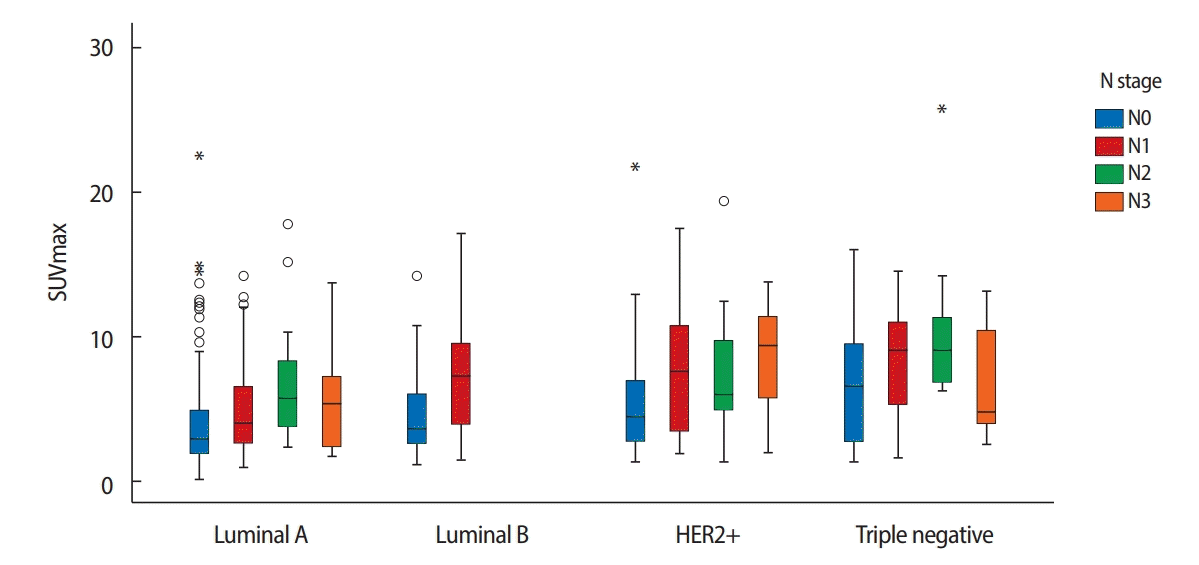
Correlation between the SUV max and axillary lymph node metastasis according to molecular subtype (p=0.001).
Cutoff SUVmax according to molecular subtypes
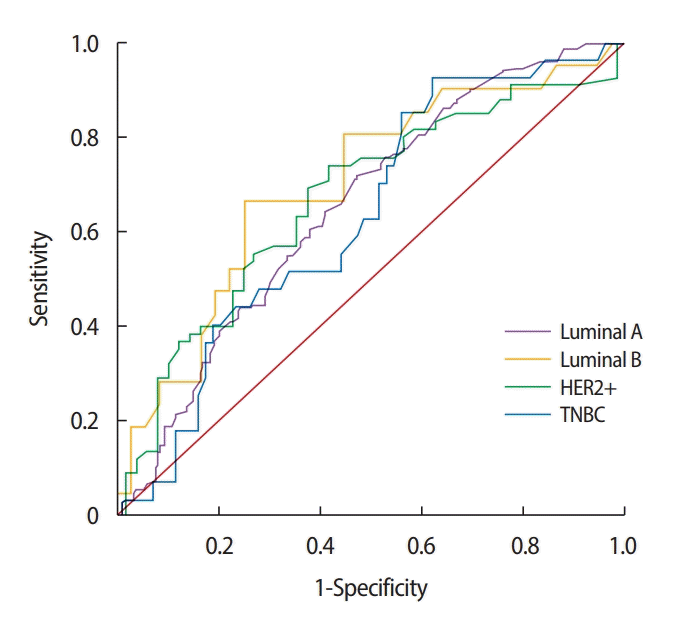
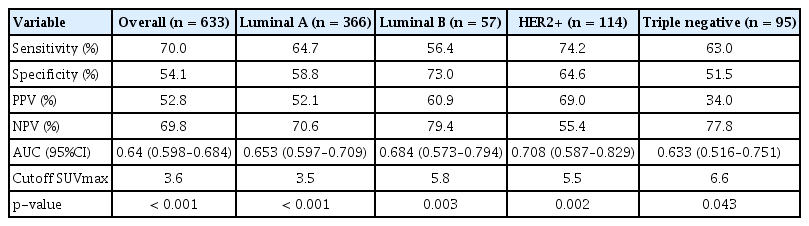
We generated the ROC curves to determine the optimal cutoff SUVmax in the detection of ALN metastasis (Figure 2). In the overall population, the AUC was 0.641 (95% confidence interval [CI], 0.598–0.684; p< 0.001), cutoff SUVmax was 3.6, sensitivity was 70.0%, and specificity was 54.1%. In the subtype analysis, the SUVmax in the HER2+ subtype predicted lymph node metastasis most accurately with an AUC of 0.708, a cutoff SUVmax of 5.5, sensitivity of 74.2%, and specificity of 64.6% (p = 0.002). In the luminal A subtype, the AUC was 0.653 (95% CI, 0.597–0.709; p< 0.001), cutoff SUVmax was 3.5, sensitivity was 64.7%, and specificity was 58.8%. In the luminal B subtype, the AUC was 0.684 (95% CI, 0.573–0.794; p = 0.003), cutoff SUVmax was 5.8, sensitivity was 56.4%, and specificity was 73.0%. In the triple negative subtype, the AUC was 0.633 (95% CI, 0.516–0.751; p=0.043), cutoff SUVmax was 6.6, sensitivity was 63.0%, and specificity was 51.5% (Table 5).
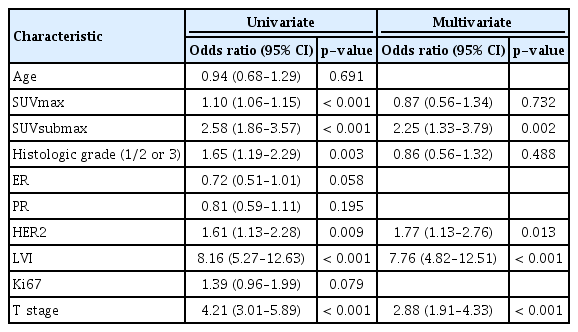
Predictors of ALN metastasis
In the univariate analysis for identifying the predictors of ALN metastasis, SUVmax, subtype-adjusted SUVmax (SUVsubmax), histological grade, HER2 positivity, LVI, and T stage were found to be significant predictors in the entire cohort. We defined the novel parameter SUVsubmax, stratifying patients above or below the subtype-optimized cut-off SUVmax (3.5 in luminal A, 5.8 in luminal B, 5.5 in HER2+, and 6.6 in triple negative). After the multivariate analysis, SUVsubmax, HER2 positivity, LVI, and T stage remained significant predictors for ALN metastasis (Table 6). In the multivariate analysis of each subtype, we included all variables that were significant in the univariate analysis, except SUVmax and HER2 positivity because these variables do not correlate well with specific subtypes. We found that SUVsubmax was a significant predictor for the HER2+ and luminal A subtypes (Table 7). However, the association was stronger for the HER2+ subtype (odds ratio [OR], 3.5; 95% CI, 1.23–9.76; p=0.019 vs. OR, 1.7; 95% CI, 1.01–2.97; p=0.045).
DISCUSSION
Our findings showed that the SUVmax of primary breast cancer was positively correlated with ALN metastasis and could serve as an independent factor for metastasis prediction. Although SUVmax could predict ALN metastasis in luminal A and luminal B subtypes, the sensitivity, specificity, and robustness in the multivariate analysis were the most accurate for the HER2+ subtype.
To date, studies on the prediction of axillary metastasis using the SUVmax of different molecular subtype tumors have provided conflicting results. The study by Kim et al., in which tumors were divided into three subtypes (ER-positive/HER2 negative, HER2 positive, and triple negative), found that a high tumor SUVmax (≥4.25) predicted ALN metastasis in ER-positive/HER2-negative and HER2-positive subtypes, but not in the triple negative subtype [11]. In contrast, one study reported that PET was the optimal tool in detecting ALN metastasis in the triple negative subtype compared to the other three subtypes, with a sensitivity of 87.0% and specificity of 67.3% [12]. However, in this study, the ratio of the ALN to the primary tumor SUVmax was used. In a study by Song et al. [13], although they established a predictive model for ALN metastasis using the cutoff SUVmax adjusted for the molecular subtypes, subtype-specific predictive values were not included.
In our study, we found that the prediction of ALN metastasis in the triple negative subtype was not as effective as that in other subtypes. Although we could predict a statistically robust ROC curve and significant cutoff SUVmax (p=0.043) in the triple negative subtype, the comparison between patients with or without metastasis did not show a statistically significantly different SUVmax (p=0.130). This may be caused by the relatively smaller number of triple negative breast cancer patients with axillary metastasis (N=27, 28.4%). However, our findings are consistent with those of other studies indicating a lower risk of ALN metastasis in triple negative breast cancer [14]. Similar to our study, a large-scale Chinese study demonstrated that the probability of lymph node metastasis in the triple negative subtype was 28% compared with 45% in other subtypes [15]. This observation probably reflects the distinct biology of this subtype, in which hematogenous spread is predominant over lymphatic spread [16]. Consequently, a different approach may be considered for patients with this subtype.
Despite conflicting existing results on the predictive value of SUVmax, we found that the cutoff SUVmax should be adjusted for each molecular subtype: a higher cutoff SUV is needed for more aggressive subtypes than that for less aggressive subtypes. Traditionally, a high SUVmax is related to poor prognostic indices, such as tumor grade, high proliferation index, ER negativity, and HER2 overexpression [17-19]. Our results are consistent with those of previous studies, showing that, in more aggressive subtypes, such as HER2+ or triple negative, a higher SUVmax is observed [20-22]. Moreover, we found that SUVsubmax (SUVmax adjusted for molecular subtypes) was a more powerful predictor for ALN metastasis than SUVmax alone. This result suggests the necessity of establishing different cutoff SUVmax values for each molecular subtype. In one study addressing this issue, cutoff SUVmax values of 3.3, 5.5, 12.7, and 11.5 were reported for luminal A, luminal B, HER2+, and triple negative subtypes, respectively [13].
Among the predictors of ALN metastasis, LVI had the greatest OR of 7.76 (95% CI, 4.82–12.51), followed by tumor size, with an OR of 2.88 (95% CI, 1.91–4.33), and SUVsubmax, with an OR of 2.25 (95% CI, 1.33–3.79). Considering that LVI and tumor size could only be accurately measured after surgery, SUVsubmax could be a useful supplementary parameter to improve the diagnostic value of PET/CT for axillary node status preoperatively.
We did not analyze the SUVmax of the ALNs. Some investigators have reported that the SUVmax of ALN (SUVmax LN) is also related to the avidity of the primary tumor, and the combined measure of SUVmax LN and primary tumor’s SUVmax may be superior to SUVmax alone [13,23-25]. However, in this study, we tried to focus on the direct association between the tumor SUVmax and ALN status because there is no reliable threshold for SUVmax LN to discriminate benign from malignant lymph nodes presently [26]. In our study, the sensitivity and specificity for the detection of ALN metastasis in the HER2+ subtype were 74.2% and 64.6%, respectively. The sensitivity of PET/CT in predicting ALN metastasis ranges from 33% to 94% in previous studies, while the specificity is relatively higher, ranging from 66% to 100% [27]. As such, the role of PET/CT in assessing the axillary status is controversial in the field. However, as PET/CT may reflect the tumor biology, integration of the biologic information provided by PET/CT to conventional diagnostic tools may potentially expand the use of PET/CT [28].
A limitation of our study is that it was a single-center retrospective study, which might have resulted in selection bias. Moreover, as there are no standards for optimal SUVmax measurement, our results must be interpreted with care, as SUV measurement protocols may differ between institutions and may be affected by multiple factors, including blood glucose level, body composition, and partial volume effect [25]. Thus, further prospective studies with a larger cohort are required to validate our results.
In conclusion, the present study revealed that the SUVmax of primary breast cancer may be an independent predictor of ALN metastasis, most accurately in the HER2+ subtype. Our results support the preoperative use of PET/CT to facilitate a tailored axillary management for initial staging determination and treatment strategy planning in patients with breast cancer.
Notes
The authors declare that they have no competing interests.