Clinical Implication of Intraoperative Sonography in Localized Excision Biopsy for Mammographic Microcalcifications
Article information
Abstract
Purpose
Ultrasonography plays a supplementary role in detecting breast microcalcifications as localizing these microcalcifications without mammographic aid is not always successful. This study aimed to evaluate the clinical implications of intraoperative sonography (IOUSG) in localized excisions after mammographically guided wire insertion.
Methods
Between May 2011 and December 2017, 90 localized excisional biopsies were included. All excisions were preceded by mammographically guided wire insertion. We divided them into two groups according to the use of IOUSG and compared the surgical outcomes between the two groups.
Results
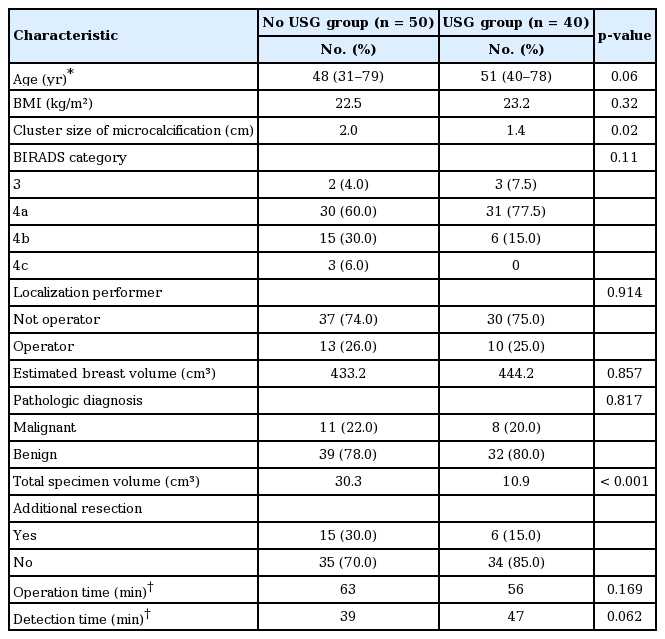
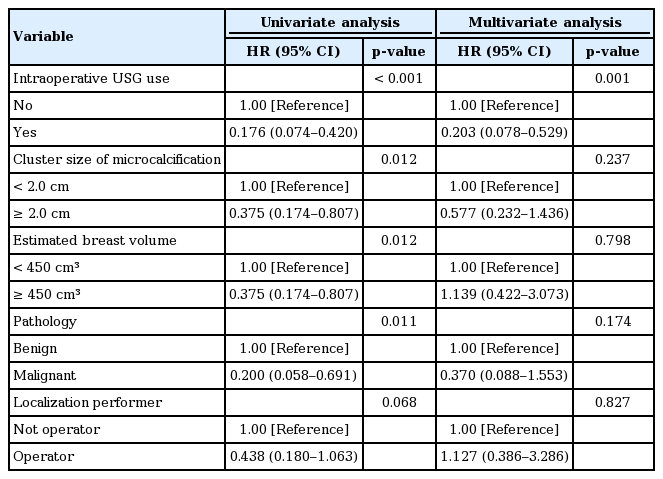
Of the 90 localized excisions analyzed, IOUSG was performed in 40 (the USG group) localized excisions and not in the remaining 50 (the no USG group) localized excisions. The median cluster size of the target microcalcifications and the median specimen volume were smaller in the USG group than that in the no USG group (1.4 cm vs. 2.0 cm, p=0.02; 10.9 cm3 vs. 30.3 cm3, p<0.001, respectively). Additional excisions due to the incomplete coverage of the target microcalcifications on the specimen mammography were more frequent in the no USG group than in the USG group (30% vs. 15%, respectively, p<0.001). In the multivariate analyses, performing an IOUSG was the only significant risk factor, reducing the need for additional excision after adjusting the other risk factors (adjusted hazard ratio, 0.203; 95% confidence interval, 0.078–0.529). Performing an IOUSG significantly reduced the specimen volume excised after adjusting the cluster size of the microcalcifications.
Conclusion
IOUSG could be helpful in improving the accuracy of surgical excision for breast microcalcifications localized with mammographically guided wire insertion.
INTRODUCTION
Mammographic microcalcification is one of the typical abnormal mammographic findings in breast cancers. Depending on the individual features of the finding, a biopsy may be required for pathologic confirmation. Notably, for some intraductal breast cancers, suspicious mammographic microcalcifications may be the only abnormal findings present, without any palpable lesions or corresponding sonographic abnormalities [1-3].
With advances in vacuum-assisted biopsy devices, stereotactic biopsy methods are being performed to assess mammographic microcalcifications. However, under the Korean National Health Insurance System, the stereotactic biopsy methods cost two to three times more than that of localized excision. Furthermore, for thin women with small breasts, using stereotactic biopsy devices can be challenging [4,5]. Therefore, localized excision after mammographically guided wire insertion is still preferred over the stereotactic methods among many physicians.
Sonographic examination for suspicious mammographic microcalcifications is known to be usually ineffective [6,7]. Microcalcifications can be easily detectable on sonography when they are seen as distinct echogenic specular reflectors, contrasting with a background hypoechoic mass or duct-like structure that increases microcalcification conspicuity. However, they are difficult to detect when they appear as broader echogenic regions without a significant mass or ductal change. Small regions of fine microcalcifications are even more difficult to detect by sonography alone.
Currently, close sonographic examination performed by an experienced sonographer using high-resolution probes allows a better visualization of breast microcalcifications [6-9]. Nonetheless, sonography remains to play a supplementary role in detecting breast microcalcifications as localizing these microcalcifications without mammographic aid is not always successful [7,10,11]. In our institution, sonography is reevaluated in some localized excision after mammographically guided wire insertion to confirm the direction and depth of the wire. We have experienced that microcalcifications are better detected by intraoperative sonography within the breast parenchyma adjacent to the wire. In this regard, we hypothesized that performing intraoperative sonography after mammographically guided wire insertion in detecting microcalcifications gives additional localizing information to the operator and provides better surgical outcomes.
The present study aimed to evaluate the clinical implications of intraoperative sonography in localized excision after mammographically guided wire insertion.
METHODS
Patients who underwent excisional biopsies due to indeterminate microcalcifications on their mammography were included in this study. Mammography was performed using a Selena device (Hologic, Marlborough, USA), and an open localization paddle was selected for compression during wire localization. Accura BLN (Argon, Frisco, USA) was used for the localizing wire. Wire localization was performed by three radiologists and two surgeons. In cases where a surgeon performed wire localization, the same surgeon also performed the surgical excision. Intraoperative sonography was performed by an operator using a high-resolution ultrasound unit with a 13-MHz linear array transducer (EUB-7500; Hitachi, Tokyo, Japan). Intraoperative sonography was performed to directly visualize the target microcalcification with the aid of localizing wire. The secondary locational information of microcalcification was used for a more precise excision.
We reviewed the medical information of the patients, which included age, gender, body mass index, Breast Imaging Reporting and Data System category of preoperative mammographic images, wire localization performers, operation time, estimated specimen volume (length×width× thickness, cm3), whether or not additional excision was required, whether or not intraoperative sonography was performed, and the postoperative pathological diagnosis.
Additionally, we reviewed the patients’ mammography films to confirm the cluster size of the target microcalcification and calculate the estimated breast volume. The following formula was recommended by Kalbhen et al. [12] and was used to estimate the breast volume: (breast volume [V] [cm³]= 0.785× height [H] [cm]×width [W] [cm]×compression thickness [C] [cm]). This formula represents the breast volume as a half elliptical cylinder in the craniocaudal projection, where H is the posterior-to-anterior height measured perpendicular from the posterior film edge to the most anterior portion of the breast (usually the nipple-areolar complex), W is the medial-to-lateral width measured along the posterior film edge, and C is the compression thickness of the breast in the craniocaudal view.
Nonparametric statistical analyses (the Mann-Whitney U-test and the chi-squared test with Fisher’s exact test) were used to compare the clinical characteristics and surgical outcomes from the excision cases in which intraoperative sonography was performed after wire localization and those from other cases in which intraoperative sonography was not performed. A linear regression model and Cox proportional hazards regression model were used to perform multivariate analyses and confirm the statistical significance. All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, USA). Two-sided p< 0.05 was considered statistically significant.
This study was approved by Institutional Review Board (IRB; No. 2018-08-004) at the Seoul Medical Center of Korea and was conducted in accordance with local regulations.
RESULTS
Between May 2011 and December 2017, 90 localized excisional biopsies were performed in 85 female patients. Among them, four patients underwent multiple localized excisional biopsies at once, and two patients underwent localized excision on one breast and breast cancer surgery on the contralateral breast, respectively. Sonography was usually reevaluated when there was difficulty in detecting the location of the target microcalcification because of long intraparenchymal wire length and/or postprocedural wire dislocation. The pathologic diagnoses of the 90 localized excisions were summarized in supplementary Table S1.
Of the 90 localized excisions analyzed, intraoperative sonography was performed in 40 (the USG group) localized excisions and not in the remaining 50 (the no USG group) localized excisions. The baseline characteristics of both groups are summarized in Table 1. Preoperative sonographic evaluations were performed in 69 cases (77%) among the 90 localized excisions to determine whether target microcalcification could be biopsied by a sonography alone. All of them failed to obtain a reliable finding when localizing the target microcalcification by a sonography-guided biopsy alone.
The median cluster size of the target microcalcification and the median specimen volume were smaller in the USG group than that in the no USG group (1.4 cm vs. 2.0 cm, respectively, p = 0.02; 10.9 cm3 vs. 30.3 cm3, respectively, p< 0.001). Additional excisions due to the incomplete coverage of the target microcalcification on the specimen mammography were more frequent in the no USG group than that in the USG group (30% vs. 15%, respectively, p< 0.001).
“Detection time” was previously defined as the duration from the time of incision to when the specimen’s suitability was confirmed by final specimen mammography [13], which more directly represents the influence of the new intervention during localized excision for mammographic microcalcifications. In the present study, both the operation time and detection time were not different between the two groups.
To determine the influence of intraoperative sonographic examination, differences in the specimen volumes and additional excision rates were reevaluated by performing multivariate analyses. Performing intraoperative sonography resulted in the following: less frequent additional excisions, larger cluster sizes of the target microcalcification (≥ 2.0 cm), larger breast volumes (≥450 cm3), and malignant pathology via univariate analyses. Additionally, when the wire localization was performed by the operator, the additional excision rate was marginally reduced. However, performing an intraoperative sonography was the only significant risk factor, reducing the need for additional excision after adjusting these variables (adjusted hazard ratio, 0.203; 95% confidence interval, 0.078–0.529) (Table 2). Similarly, the influence of performing an intraoperative sonography to specimen volume excised was reexamined via a multivariate analysis using a linear regression model. The larger cluster size of microcalcifications (≥ 2.0 cm) significantly increased the specimen volume excised. On the other hand, performing an intraoperative sonography significantly reduced the specimen volume (Table 3).
DISCUSSION
We aimed to determine whether performing an intraoperative sonography after mammographically guided wire insertion could result in better surgical outcomes in localized excision in detecting suspicious mammographic microcalcifications. We hypothesized that additional sonography would provide more localizing information on microcalcifications and make the excision more precise. Analyzing the surgical outcomes of the localized excisions for breast microcalcifications, we noted that the target microcalcification was retrieved with a smaller volume of specimen in the USG group than that in the no USG group. Furthermore, additional excisions, resulting from the failure of the first excision to successfully localize the target microcalcifications, were less frequent in the USG group than that in the no USG group (Table 2, 3).
Unlike en bloc excisions for malignant diseases, localized excision for breast microcalcifications is performed to establish an accurate pathologic diagnosis with a minimal volume of excised specimen. Regarding a group of suspicious breast microcalcifications, a wider excision can improve the accuracy of the localized excision because a larger specimen includes target microcalcification more easily. However, in this study, additional excisions were less frequent in the USG group even though the excised specimen volume was smaller in this group. These findings support the clinical usefulness of performing an intraoperative sonography during diagnostic surgical excisions for breast microcalcifications.
The size of the specimen excised in a diagnostic surgical excision is largely determined by the size of the target lesion. In this study, the median cluster size of the target microcalcification was smaller in the USG group than that in the no USG group (1.4 cm vs. 2.0 cm, respectively, p< 0.001; Table 1). Therefore, that variable can act as a confounding factor in comparing the specimen volumes excised between the two groups. In the multivariate analyses comparing the differences in specimen volumes between the two groups, performing an intraoperative sonography was an independent risk factor, reducing the specimen volumes excised after adjusting the cluster size of the target microcalcifications (Table 3). On the other hand, the additional excision rate was not different by the cluster size of the microcalcifications (Table 2).
In the previous studies, the reported detection rates of breast microcalcifications on sonography ranged from 20% to 35% [7,11,14,15]. The detection rates were much lower in cases that had no sonographically detectable masses associated with microcalcifications as in most cases in this study, which ranged from 5% to 27%. Over the past two decades, great strides have been made in improving the resolution and contrast in breast sonography, allowing better and more frequent visualization of breast microcalcifications [6-9]. Most breast microcalcifications might be detected on sonography at present if an experienced sonographer examines them for a long enough time using a high-resolution ultrasound unit. Indeed, the problem in this regard is whether one can retain reliable accuracy. However, a recent study that reviewed over 10,000 diagnostic mammographies reported that only 87 (22%) among the 391 lesions that had suspicious mammographic microcalcifications without associated findings could be percutaneously biopsied by sonography only [10]. Unfortunately, sonography alone seems to have an insufficient reliability in detecting breast microcalcifications until now.
To enhance the performance of sonography in localizing breast microcalcifications, there have been attempts at combining USG with other localizing interventions. Choo et al. [16] reported a high retrieval rate (97%) of a combination of wire localization and ultrasound-guided, vacuum-assisted breast biopsy for clustered microcalcifications. However, they performed a sonography to locate the wire without mentioning the visibility of the microcalcifications. Herein, we performed an intraoperative sonography as an additional method to further localize the target microcalcification by a focused examination around the localizing wire. This different performance of sonography might warrant better reproducibility for experienced sonographers to find a proper target region. In the USG group in the present study, all of the target microcalcifications could be confirmed or at least highly suspected to a specific region by examining the breast parenchyma adjacent to the localizing wires. The two surgeons in the present study are equally specializing in breast surgery and had breast ultrasonography experience for 2 and 4 years, respectively. Breast surgeons’ complete understanding of the breast anatomy would allow them to have a better comprehension of the vague sonographic conspicuity of the target microcalcification.
In our previous work, we reported that the “detection time” in surgical excisions after mammographically guided wire insertion was shorter when the operators performed wire localizations themselves [13]. Analyzing that result, we had suspected that other surgical outcomes such as excised specimen volumes and additional excision rates might be affected by the wire localization performers. However, in this study, there was no difference in the wire localization performers between the USG and no USG groups. Additionally, the specimen volumes excised and the additional excision rates did not differ between the wire localization performers (Table 2, 3).
Our study has several limitations. First, possible confounders might be missed in this study because of its retrospective nature. We compensated that limitation by performing multivariable analyses including almost all possible risk factors with clinical experience as surgical sonographers and related literature review. Another limitation is in our incomplete documentation for the required number of years of experience for our surgical sonographers. Evaluating microcalcification with breast sonography might be challenging to a beginner. However, sonography is not an unfamiliar skill to surgeons anymore because many surgeons in Korea are interested in sonography as a supplementary tool.
Apart from being a powerful diagnostic tool, ultrasonography is being widely performed as an interventional procedure for breast diseases. In the present study, we noted that intraoperative ultrasonography could be helpful to improve the accuracy of surgical excision for breast microcalcifications localized with mammographically guided wire insertion. This useful tool is expected to be used together with various interventional methods with advances in ultrasonographic technology.
Supplementary Materials
Pathologic diagnoses of the 90 localized excisions for breast microcalcifications
Notes
The authors declare that they have no competing interests.