Apoptosis and Cell Cycle Arrest in Two Human Breast Cancer Cell Lines by Dieckol Isolated from Ecklonia cava
Article information
Abstract
Purpose
Dieckol, a phlorotannin compound isolated from Ecklonia cava, has been reported to have antioxidant, antiviral, anti-inflammatory, and anticancer properties. The purpose of this study was to investigate its anticancer effects on human breast cancer cell lines.
Methods
In this study, the viability of two human breast cancer cell lines SK-BR-3 and MCF-7 was investigated after dieckol treatment using a WST-1 assay. Apoptosis and cell cycle distribution were assayed via Annexin V-fluorescein isothiocyanate and propidium iodide staining followed by flow cytometric analysis. Immunoblotting analysis was also performed using Bax/Bcl-2 to determine whether the dieckol-induced apoptosis was mediated by the intrinsic apoptotic pathway.
Results
In a dose dependent manner, dieckol reduced the number of viable cells and increased the number of apoptotic cells. The effect of dieckol on the cell cycle distribution was analyzed using flow cytometry. Dieckol treatment significantly increased the percentage of MCF-7 and SK-BR-3 in the G2/M phase. Immunoblot analysis revealed that 24 hours of dieckol exposure increased the Bax/Bcl-2 ratio.
Conclusion
Dieckol induced cytotoxicity in MCF-7 and SK-BR-3 human breast cancer cells inducing apoptosis and cell cycle arrest. Therefore, it is suggested that dieckol may be a potential therapeutic agent for breast cancer.
INTRODUCTION
With advances in treatment, earlier detection, and the increased awareness of health care, the mortality rate due to breast cancer has been decreasing over the last few decades [1]. However, breast cancer is still a major cause of morbidity and mortality among women worldwide. Moreover, it is the second most common cancer in Korean women [2]. Generally, the treatment of breast cancer consists of surgery, chemotherapy, radiation therapy, antihormonal therapy, and/or targeted therapy (trastuzumab). These treatment strategies vary for each patient, depending on the breast cancer subtype. Since breast cancer is a disease characterized by multiple subtypes, there are differences in disease prognosis and the response to diverse therapeutic options [3-5]. The recurrence of breast cancer can occur at any time, from months to years after the initial treatment. Late recurrence is not common in breast cancer but may arise at any stage [6]. Prognosis is poor in patients with recurrent or metastatic breast cancer, despite adequate treatments such as chemotherapy or radiation therapy. Additionally, cancer treatment is known to cause adverse effects including febrile neutropenia, hair loss, peripheral neuropathy, ototoxicity, and nephrotoxicity [7-9]. These events decrease the patient’s compliance to treatment. Therefore, new therapeutic options are needed with consideration of the patient’s compliance and quality of life.
Extensive research has revealed that numerous natural products, especially those obtained from aquatic plants, have potential chemopreventive and antineoplastic effects on breast cancer [10,11]. Among these, brown algae, i.e., Ecklonia cava, are used as health foods in East Asia. In addition, they are used in the production of many food products [12,13]. E. cava contains various natural components, such as carotenoids, fucoidans, and phlorotannins. Dieckol, a phlorotannin from E. cava has antioxidant, antithrombotic, profibrinolytic properties [14,15]. Recent studies have reported that this phlorotannin has an inhibitory effect on cancer metabolism [16,17]. However, the effect of dieckol on cancer cell growth has not been extensively studied in breast cancer.
Therefore, in this study, the effect of dieckol isolated from E. cava was evaluated on human breast cancer cell growth. Further, its mechanism of action on the cell lines, i.e., induction of apoptosis and/or cell cycle arrest was investigated.
METHODS
Cell lines and culture
Human breast adenocarcinoma (MCF-7 and SK-BR-3) cells were purchased from the American Tissue Culture Collection (ATCC, Manassas, USA). These cells were cultured in Dulbecco’s modified Eagle medium: Nutrient Mixture F-12 (DMEM/F-12) supplemented with 10% fetal bovine serum and 1% Penicillin-Streptomycin. The cells were maintained at 37°C in a 5% CO2 incubator. Dieckol was solubilized in dimethyl sulfoxide and then diluted in DMEM/F-12 medium for cell treatment.
Chemical treatment and cell viability assay
MCF-7 and SK-BR-3 cells were seeded in 96-well plates at a density of 1,000 cells/well in 100 μL of medium and permitted to adhere for 24 hours at 37°C. The cells were treated with various concentrations of the compound (25, 50, 100, 200, and 400 μg/mL) and incubated for 24 hours at 37°C. The cell viability was evaluated using a WST-1 kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s protocol. In brief, 10 μL of the WST-1 reagent was added to the wells, and the cells were incubated at 37°C for 3 hours. The cells were analyzed using a plate reader at a wavelength of 440 nm. The results are from experiments repeated at least in triplicate.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot assay
The protein samples were balanced, and approximately 20 μg of protein was separated on 10% to 18% acrylamide gels and then transferred to polyvinylidene difluoride membranes (GE Healthcare, Mississauga, Canada). The membranes were blocked with 5% nonfat dried milk for 1 hour and incubated overnight at 4°C with the appropriate primary antibodies (Sigma Aldrich, Cell Signaling, Darmstadt, Germany). The secondary antibodies used were either goat anti-rabbit or anti-mouse IgG (Bio-Rad, Seoul, Korea) conjugated to horseradish peroxidase. A chemiluminescent substrate (GE Healthcare) was used to visualize the immunoreactive proteins.
Annexin V and propidium iodide double staining for the analysis of apoptosis
For the analysis of cell apoptosis, cells were treated with 100 μg/mL of dieckol for 24 hours at 37°C. The cells were collected and stained using the fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit I (BD Biosciences, Seoul, Korea) according to the manufacturer’s protocol. In brief, the cells were seeded in 6-well plates at a density of 3×105 cells/well in 2 mL of medium and were permitted to adhere for 24 hours at 37°C. The cells were treated with dieckol and incubated for 24 hours at 37°C. The cells were harvested in a 5-mL flow cytometry (FACS) tube and washed with cold phosphate-buffered saline (PBS). FITC-Annexin V and propidium iodide (PI) were both added, and the solution incubated for 15 minutes at room temperature (25°C) in the dark. Samples were analyzed by FACS (BD Biosciences).
Assays of cell cycle
For the analysis of the cell cycle, the dieckol-treated cells were collected and fixed with 70% ice-cold ethanol for 1 hour at 4°C. The cells were subsequently washed once with PBS. They were then incubated in PBS with RNase (0.5 mg/mL) for 1 hour at 37°C. Further, the cells were then incubated with PI for 30 minutes at room temperature (25°C) in the dark. The samples were analyzed by FACS (BD Biosciences) at 488 nm.
RESULTS
Dieckol suppresses breast cancer cell growth
The inhibitory effect of dieckol on MCF-7 and SK-BR-3 cell growth was investigated. These cells were exposed to various concentrations of dieckol for 24 hours, and the cell viability was assessed using a WST-1 assay. The results showed a dose-dependent effect on cell viability. Cell death reached 57.55% with 400 μg/mL of dieckol and 15.40% with 200 μg/mL of dieckol after 24 hours in MCF-7 cells. Similar results were observed in the SK-BR-3 cells. Cell death reached 63.29% with 400 μg/mL of dieckol and 40.89% with 200 μg/mL of dieckol in comparison with the control (Figure 1).
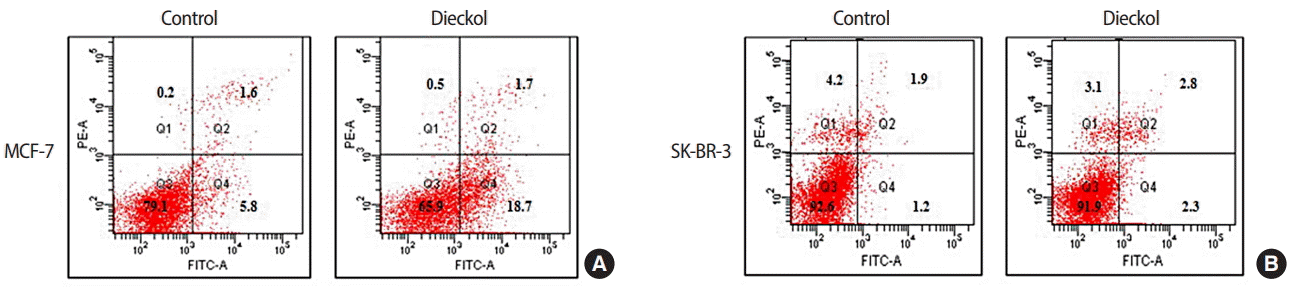
Apoptosis induced by dieckol
To investigate the mechanism of the cell death induced by this compound, apoptosis was analyzed. The results are presented in Figure 2. These cells were treated with a fixed concentration of dieckol (100 μg/mL). Flow cytometric assay showed different results between the cell lines. Dieckol-induced apoptosis was significantly higher in MCF-7 cells than in SK-BR-3 cells. The percentage of apoptotic cells increased from 5.8% to 18.7% in the MCF-7 cell line and from 1.2% to 2.3% in the SK-BR-3 cell line.
Bax/Bcl-2 ratio in the breast cancer cell lines treated with dieckol
To determine the amount of dieckol-induced apoptosis through the modulation of the Bcl-2 proteins, the Bax/Bcl-2 ratios were measured at the protein level. These ratios in the MCF-7 and SK-BR-3 cells were determined at 24 and 48 hours after treatment with dieckol (100 μg/mL). The expression of β-actin (42 kDa) was used as a loading control. The Bax/Bcl-2 ratio was higher in the dieckol-treated group than in the control at 24 hours (p< 0.001) (Figure 3A). However, it was observed that in the MCF-7 cell line, this ratio was not significantly different between the control and the dieckol-treated group at 48 hours. The ratios measured in SK-BR-3 cells are displayed in Figure 3B. There was a significant difference (p< 0.001) in the Bax/Bcl-2 ratio between the cells treated with dieckol for 24 and 48 hours.
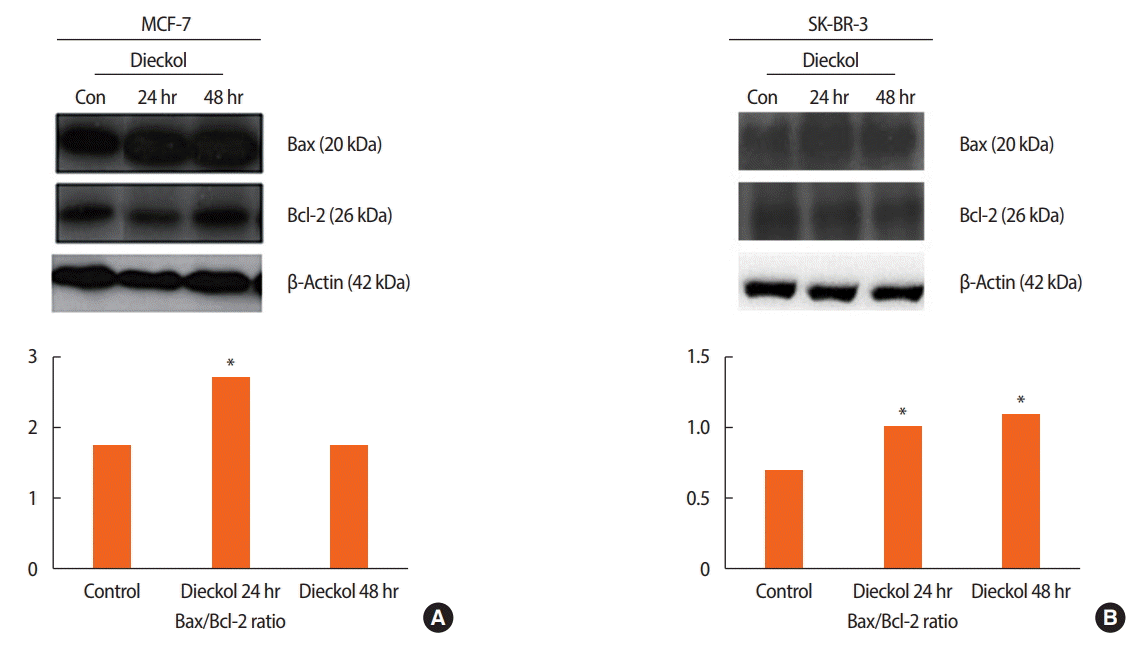
Bax/Bcl-2 ratio measured by western blot analysis. Three groups are shown: control (con); 100 µg/mL dieckol for 24 hours; 100 µg/mL dieckol for 48 hours. (A) In MCF-7 cell, representative gel pictures showing level of expression Bax, Bcl-2, and β-actin densitometric analysis showing three groups. (B) In SKBR-3 cell, representative gel pictures showing level of expression of Bax, Bcl-2, and β-actin densitometric analysis showing three groups.
*Significant difference from control p<0.001.
Cell cycle arrested induced by dieckol
The effect of dieckol on the cell cycle distribution was analyzed using flow cytometry. As shown in Figure 4A, in the dieckol-treated groups, the percentage of MCF-7 cells in the S phase decreased. On the other hand, there was an increase in the percentage of cells in the G2/M phase. These results showed that dieckol induced cell cycle arrest at the G2/M phase in MCF-7 cells.
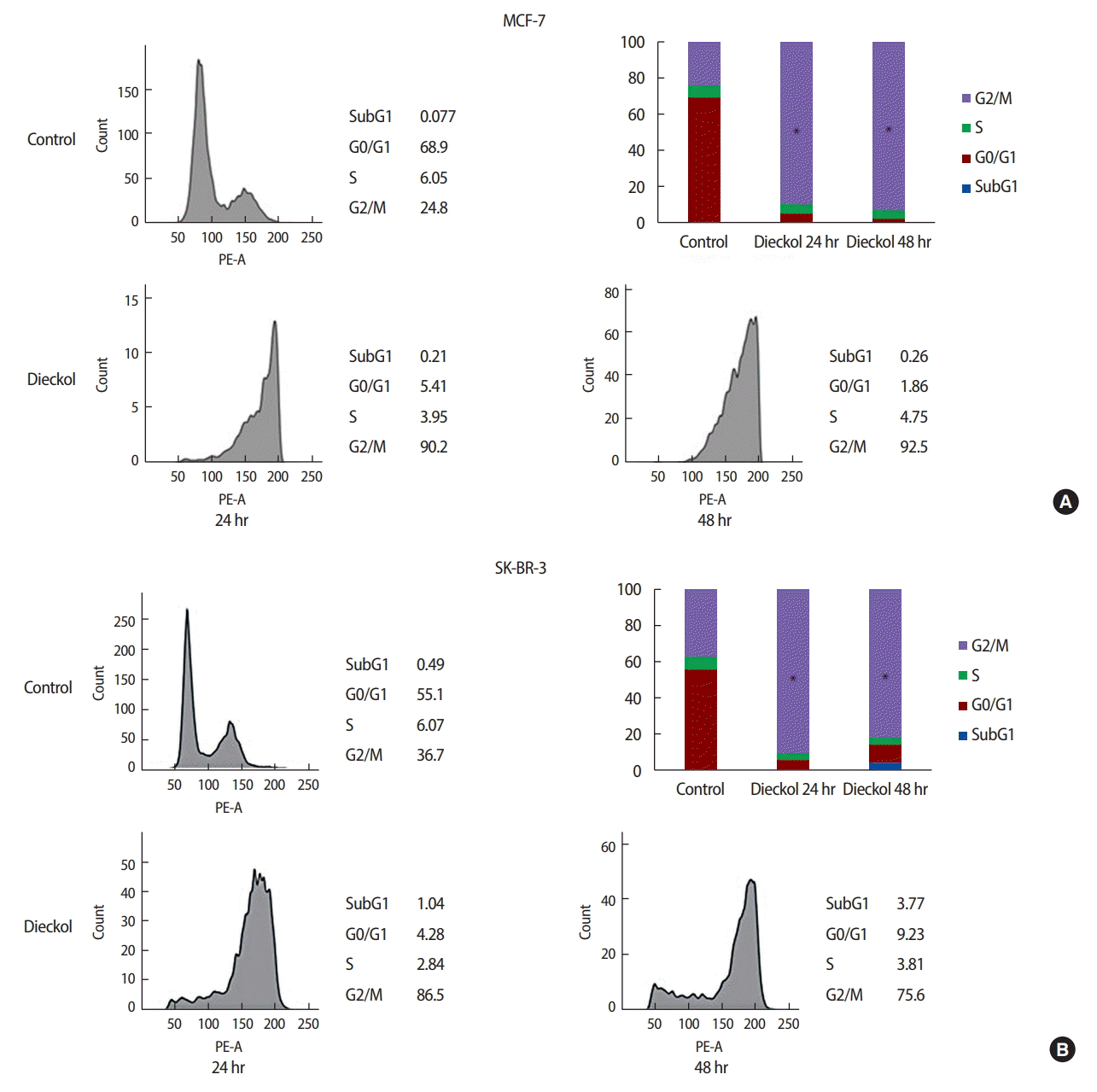
Dieckol induces cell cycle arrest at the G2/M phase in MCF-7 (A) and SK-BR-3 (B) cells. The cell cycle was measured by flow cytometry.
PE-A=phycoerythrin. *Significant difference from control p<0.001.
Figure 4B shows the results of the cell cycle analysis of the SK-BR-3 cells with and without dieckol treatment. Compared to the control group, the dieckol significantly increased the percentage of SK-BR-3 cells in the G2/M phase.
DISCUSSION
Marine brown algae, E. cava, are abundant on the Jeju island of Korea and Japan. This species has been found to contain various phlorotannins, such as fucodiphlorethol G, 7-phloro eckol, 6,6’-bieckol, eckol, dieckol, and phlorofucofuroeckol A [18-20]. These components are known to have strong antioxidant, antibacterial, anti-inflammatory, antidiabetic, and anticancer activities [16,21-23]. Most studies related to the anticancer effect of dieckol have been performed by Korean researchers. This phenomenon may occur because it dieckol is an affordable ingredient in Korea. However, studies regarding its anticancer effects have not been reported. To the best of our knowledge, this is the first study that has investigated the anticancer effects of dieckol, such as apoptosis and cell cycle arrest, using two breast cancer cell lines (MCF-7 and SK-BR-3). These cells exhibit different phenotypes of breast cancer. MCF-7 is hormone-receptor positive and human epidermal growth factor receptor 2 (HER2) negative, whereas SK-BR-3 is hormone-receptor positive and overexpresses HER2. Although MCF-7 and SK-BR-3 are different, the anticancer effect of dieckol did not vary between the two cell lines.
In this study, since the exact dieckol concentration required to cause cancer cell death was unknown, various concentrations were applied. It was observed that dieckol was highly effective at killing the MCF-7 and SK-BR-3 breast cancer cell lines in a dose-dependent manner. There was a significant decrease in the proliferation of both the cancer cell lines treated with 200 μg/mL of dieckol for 24 hours compared to the control. Therefore, dieckol could cause cytotoxicity in breast cancer cells.
Apoptosis consists of two major pathways, the extrinsic and intrinsic pathways. The intrinsic pathway, i.e., the mitochondrial-mediated pathway, is activated by various stimuli, such as reactive oxygen species, DNA damage, calcium, and Bcl-2 antiapoptotic proteins. Moreover, the mitochondrial-mediated pathway is regulated by the antiapoptotic Bcl-2 family and the proapoptotic Bax protein. Bcl-2 inhibits apoptosis by interacting with and forming inactivating heterodimers with Bax/Bak [24,25]. To investigate whether apoptosis was induced by the mitochondrial-mediated pathway, the Bax/Bcl-2 ratio in the breast cancer cell lines treated with dieckol was measured. It was revealed that the Bax/Bcl-2 ratio increased in both cell lines treated with dieckol for 24 hours. These results indicate that the dieckol-induced apoptosis may be initiated by the mitochondrial pathway. Interestingly. there was no increase in the Bax/Bcl-2 ratio in the MCF-7 cells treated with dieckol for 48 hours, which is in contrast with the result of SK-BR-3 cells treated for the same amount of time. The reason for this discrepancy in the Bax/Bcl-2 ratio in both cell lines treated with dieckol for 48 hours could be because they are different cell types. However, this finding warrants further investigation.
The cell cycle is a set of events responsible for cell replication [26]. In normal cells, the cell cycle is controlled by various signaling pathways that detect cellular events, such as cellular stress or DNA damage. These pathways are generally referred to as cell cycle checkpoints. These checkpoints play a role in correcting and repairing cellular damage, cell cycle arrest, and apoptosis [27,28]. In tumor metabolism, uncontrolled cell proliferation is observed because of malfunctions in the regulatory processes. In addition, evading cell cycle arrest is the most frequently observed phenomenon. Checkpoints at the G1/S and G2/M transitions are crucial to cell cycle progression without accumulation of genetic abnormalities [29,30]. In this study, the MCF-7 and SK-BR-3 cells treated with a concentration of 100 μg/mL of dieckol for 48 hours were arrested at the G2/M phase. These findings indicate that the dieckol-induced cell cycle arrest occurred regardless of the cell line. However, the precise mechanisms of the cell cycle arrest in the MCF-7 and SK-BR-3 cells are unknown. Therefore, further investigation is needed.
In conclusion, this study indicated that dieckol suppresses cell proliferation, activates the intrinsic apoptotic pathway, and induces cell cycle arrest at the G2/M phase. Although further investigations are required to be conducted in vivo, these findings can support the development of dieckol as a potential agent for breast cancer treatment.
Notes
The authors declare that they have no competing interests.
Acknowledgements
Dieckol was supported by Botamedi, Inc., Seoul, Korea