Hormonal Receptor Positivity and Clinical Multifocality Are Independent Predisposing Factors for Positive Margin Events in Breast-Conserving Surgery after Neoadjuvant Chemotherapy
Article information
Abstract
Purpose:
Neoadjuvant chemotherapy (NAC) frequently results in shrinkage of the primary tumor. It is not easy to perform breast-conserving surgery (BCS) after NAC, based on tumor extent alone. We identified the clinicopathological factors associated with positive margins on frozen or permanent sections in patients undergoing BCS after NAC.
Methods:
The records of 151 patients who had BCS after NAC between 2005 and 2010 were reviewed. All patients underwent subsequent imaging work-up including breast magnetic resonance imaging, ultrasound, and breast mammography at the midpoint and/or the end of NAC. Positive resection margins on frozen or permanent sections were considered to be due to the presence of either invasive carcinoma or in situ carcinoma. The relationship between the microscopic margin status and clinicopathological factors was analyzed when positive margins were detected.
Results:
Of 151 patients, 39 (25.8%) were diagnosed with a pathological complete response, while 135 patients (89.4%) had a negative margin on both frozen and permanent sections and 16 (10.6%) had a positive margin on frozen or permanent sections. Of the 16 patients, 14 finally obtained negative margins after additional excision and two (1.3%) had positive margins due to in situ carcinoma. Multivariate analysis revealed that clinical multifocality after NAC (p=0.006), and hormonal receptor (HR) positivity (p=0.028) were significantly associated with positive margins on frozen or permanent sections, but were not associated with tumor size after NAC, specimen volume, or human epidermal growth factor receptor 2 positivity.
Conclusion:
We propose that HR positivity and clinical multifocality after NAC are predisposing factors for positive margins in patients undergoing BCS after NAC. It is necessary to obtain safe resection margins to avoid positive margins in these patients.
INTRODUCTION
Breast-conserving surgery (BCS) and radiation therapy are locoregional treatments that serve as alternatives to mastectomy for women with early-stage breast cancer [1]. Neoadjuvant chemotherapy (NAC) has very attractive advantages for patients with advanced breast cancer. First, it is a unique opportunity to evaluate treatment response in vivo. Second, reducing the tumor size increases the likelihood of successful BCS [2,3]. However, several authors have reported high local recurrence rates in patients who undergo BCS after NAC [4,5]; therefore, some breast oncologic surgeons are reluctant to advocate BCS after NAC.
There is ongoing controversy regarding the optimal safety margins for patients undergoing BCS, with and without NAC [6,7]. The Society of Surgical Oncology/American Society of Radiation Oncology consensus panel at the 15th annual meeting of the American Society of Breast Surgeons concluded that ‘‘no ink on the tumor’’ constituted an adequate surgical margin for BCS in patients with invasive breast cancers [8]. However, the acceptable safety margin may differ depending on whether the patient is undergoing conventional BCS or BCS after NAC. A reduction in tumor size makes identification of the original tumor size difficult, particularly when the treatment response is robust. Moreover, large tumors at presentation may not be uniformly destroyed by chemotherapy; thus, a negative resection margin is not guaranteed [9,10]. Nevertheless, the goals for the breast oncologic surgeon should be complete cancer extirpation with acceptable safety margins and an optimal cosmetic result. Positive margins in women who have received conventional BCS lead to increased ipsilateral breast tumor reappearance rates [11,12]. Herein, we identified the clinicopathological factors associated with positive margins on frozen or permanent sections in patients undergoing BCS after NAC. In addition, we identified an effective surgical strategy to avoid positive margins and to achieve good cosmetic results in these patients.
METHODS
Our Institutional Review Board (IRB) approved this retrospective study (IRB approval number: SMC-2014-10-098).
A total of 151 candidates for mastectomy with either locoregional advanced or monofocal operable tumors underwent BCS after NAC, between 2005 and 2010 at the Samsung Medical Center, Korea. All patients had documented invasive ductal carcinoma on postoperative permanent pathology or on diagnostic biopsy when no residual tumor was detected on postoperative pathology.
Hormonal receptor (HR), including estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression, was determined by immunohistochemistry (IHC). Allred scores ranging from 3 to 8 were considered to indicate positive ER and PR immunoreactivity. HER2 positivity was established based on IHC 3+ staining or amplification by fluorescence in situ hybridization. Breast magnetic resonance imaging (MRI), sonography, and mammography were employed to identify tumor size and position. Complete staging (chest radiography, liver ultrasound, and bone scintigraphy) was performed to exclude distant metastasis. All patients underwent a subsequent imaging work-up, including breast MRI, ultrasound, and breast mammography at the midpoint and/or the end of NAC. Clinical and pathological staging was carried out according to the American Joint Committee on Cancer Staging manual (7th edition) [13].
Definitions of variables
The clinical neoadjuvant response following NAC was defined by breast MRI findings based on the revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.1 [14]. A clinical complete response (cCR) was defined as no evidence of malignancy in the breast by using MRI. All other clinical responses, i.e., a partial response, stable disease, and progressive disease were defined as noncCR. A pathological complete response (pCR) was defined as no evidence of malignancy in the breast in the permanent pathological report. The clinical tumor size after NAC, including that of invasive and in situ carcinomas, was estimated along the long axis of the malignant lesion by breast MRI, sonography, and mammography. Clinical multifocality was defined as evidence of a multifocal lesion in the breast by using MRI, sonography, and mammography after NAC. A breast lesion was regarded as multifocal if more than one well-demarcated invasive or in situ tumor was detected with a mass or nonmass lesion, such as microcalcifications on mammography or other suspicious lesions on sonography or breast MRI, separated from each other, regardless of the distance between the lesions.
The BCS specimen volume was estimated by the half-ellipsoid-volume method according to the details in the pathology report (4π abc/6; where a, b, and c are ellipsoid semi-axes) [15].
Neoadjuvant chemotherapy regimens
Patients were treated with three main neoadjuvant regimens: (1) an anthracycline-containing regimen comprising 60 mg/m2 doxorubicin intravenously (i.v.) and 600 mg/m2 cyclophosphamide i.v. on day 1, every 3 weeks for 4 or 6 cycles; (2) a taxane-containing regimen comprising 50 mg/m2 doxorubicin i.v. and 75 mg/m2 docetaxel i.v. on day 1, every 3 weeks, and then, doxorubicin followed by docetaxel, for 4 or 6 cycles; and (3) an anthracycline- and taxane-containing regimen comprising 60 mg/m2 doxorubicin i.v. and 600 mg/m2 cyclophosphamide i.v. on day 1, every 3 weeks for 4 cycles, and then, after 22 days, 75 mg/m2 docetaxel i.v. was administered every 3 weeks for 4 cycles. Other NAC regimens included that of the NeoSphere trial [16], which involved trastuzumab and/or pertuzumab, plus docetaxel or trastuzumab and pertuzumab without docetaxel; doxorubicin/cyclophosphamide plus ixabepilone; the NeoALTTO trial [17] lapatinib-containing regimen; and neo-PGH trial comprising paclitaxel, gemcitabine plus trastuzumab; the HannaH study [18] regimen, which comprised docetaxel followed by fluorouracil, epirubicin, and cyclophosphamide concurrently with trastuzumab.
Adjuvant therapy
Adjuvant radiation therapy was delivered to the whole breast at a total dose of 50 Gy in 25 fractions. A tangential technique with 4- or 6-MV photon beams generated by a linear accelerator (Varian Medical Systems, Palo Alto, USA) was used for whole breast irradiation. The primary tumor bed was boosted according to the resection margin status and primary tumor size. The boost dose was 9–12 Gy with a daily dose of 2–3.5 Gy. An electron beam was used for the primary tumor bed boost. Irradiation of the supraclavicular fossa was undertaken according to clinical and pathological N stage. Adjuvant hormone therapy and HER2-targeted therapy were administered based on the IHC results.
Surgical technique and assessment of margin status
We attempted en bloc resection if there was a suspicion of invasive or in situ carcinoma. The surgical margin was deemed to be 1 cm from the tumor by preoperative modalities. Preoperative localization of a nonpalpable or a multiformly destroyed mass after NAC was performed by sonography-guided tattoo localization, wire localization, or skin marking, and metallic clip insertion. Suspicious microcalcifications were always completely excised with intraoperative specimen mammography to ensure adequate resection. Interpretation of the frozen- and permanent-surgical margins was carried out by four pathologists, specializing in breast pathology. The margin was considered positive in the presence of either invasive carcinoma or in situ carcinoma at the cut margin. The margins of the intraoperative frozen section were evaluated in six dimensions, and additional resection was performed to obtain a frozen section with negative margins. Following confirmation of negative surgical margins, breast reconstruction was carried out with consideration given to the volume and location of the removed breast tissue. When the margins were positive on the permanent section, additional excision was recommended to obtain negative permanent margins.
Statistical methods
The clinicopathological parameters associated with positive margins on frozen or permanent sections were analyzed by using the Wilcoxon two-sample test and the chi-square test. A p-value < 0.05 was considered statistically significant. A logistic regression model with Firth’s penalized maximum likelihood estimation method was used. Preoperative variables with p-values < 0.05 were selected by univariate analysis to determine predisposing factors for positive margins. Clinical T-stage at diagnosis representing the extent of the primary tumor, clinical tumor size after NAC, preoperative localization, and specimen volume were clinically important factors required to obtain negative margins, and were included in the logistic regression model. Hazard ratios and 95% confidence intervals were calculated. The statistical analysis was executed using SAS version 9.3 (SAS Institute, Cary, USA) and R 2.13.2 (Vienna, Austria; http://www.R-project.org).
RESULTS
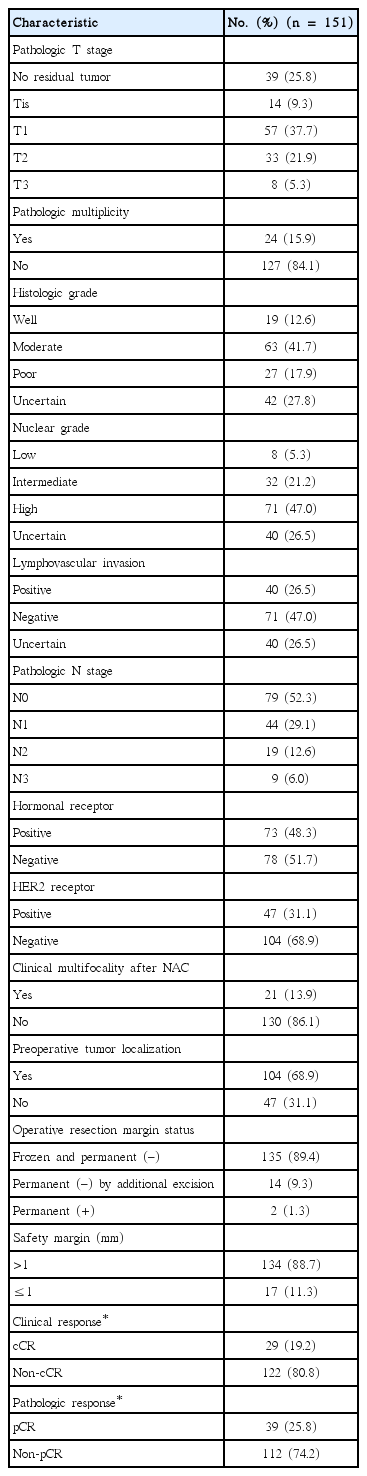
In total, 151 patients were reviewed in this analysis. The patient characteristics are shown in Table 1. The median age at diagnosis was 44 years (range, 23–72 years). The median clinical tumor size at diagnosis was 4.1 cm (range, 1–11 cm).
Fifty-six (37.1%) and 24 patients (15.9%) received anthracycline- and taxane-containing regimens, respectively, while 34 (22.5%) received a regimen containing both anthracycline and taxane. In addition, 37 patients (24.5%) underwent one of the other NAC or clinical trial regimens. Neoadjuvant HER2-targeted therapy was received by 36 of 47 (76.6%) HER2-positive patients.
Of the 151 patients, 148 (98.0%) underwent BCS and axillary lymph node dissection, and the remaining three (2.0%) underwent sentinel lymph node biopsy only. After surgery, 79 patients received adjuvant chemotherapy as stated in the initial planned schedule. A total of 148 patients underwent radiation therapy; the primary tumor bed was boosted in 123 of 148 (83.1%) patients, whereas 14 (9.3%) underwent radiation therapy elsewhere; the remaining three patients refused radiation therapy. Based on the IHC results, adjuvant hormonal and HER2-targeted therapy were administered to all 73 (100%) and 43 of 47 (91%) of the HR- and HER2-positive patients, respectively.
The clinicopathological characteristics are shown in Table 2. According to the RECIST guidelines [14], a cCR was predicted for 29 patients; however, a pCR occurred in 39 patients. A pathological partial response, stable disease, and progressive disease were recorded in 79, 29, and 4 patients, respectively. A total of 118 patients (78.1%) were downstaged, thus allowing BCS.
In total, 135 patients (89.4%) had negative margins on both frozen and permanent sections, 14 (9.3%) had initial positive margins on frozen or permanent sections, but finally obtained negative resection margins following additional excisions. Of 14 patients, 12 underwent additional intraoperative excisions and two patients underwent additional excision after initial primary BCS because of initial positive permanent-section margins. Two patients (1.3%) who had only undergone radiation therapy without additional surgery and who initially had negative margins on the frozen sections, finally had positive permanent-section margins showing in situ carcinoma. The sensitivity, specificity, and positive and negative predictive values of the frozen section biopsies were 85.7%, 98.5%, 85.7%, and 98.5%, respectively.
Clinical multifocality was reported in 21 patients (13.9%) after NAC. Preoperative tumor localization was performed in 104 patients (68.9%) including sonography-guided tattoo localization (n= 61), wire localization (n=15), skin marking (n=13), metallic clip insertion (n=1), or two or more combined localization methods (n=14).
Univariate analyses to determine predisposing factors for positive frozen- or permanent-section margins were conducted. The predictors were clinical multifocality after NAC, clinical NAC response, pathological NAC response, pathological tumor size (long axis), pathological multiplicity, and hormonal receptor status (Table 3). We selected the clinically important preoperative variables required to obtain negative margins and included them in a logistic regression model. Multivariate analyses revealed that clinical multifocality after NAC (p=0.006) and HR positivity (p=0.028) were independent risk factors for positive frozen- or permanent-section margins, but were not associated with clinical T-stage at diagnosis, tumor size after NAC, preoperative localization, specimen volume, or HER2-receptor positivity (Table 4).
DISCUSSION
Over the past decade, NAC has been increasingly used for patients with operable breast cancer to reduce large tumors to a size eligible for BCS. Clinical trials and meta-analyses have established equivalent overall survival rates for pre- and postoperative chemotherapy; however, the question of long-term local control following BCS after NAC remains controversial [2,3,19]. Although some studies have reported locoregional recurrence (LRR) rates following NAC and BCS as high as 26%, more recently reported rates are 7% to 16% [2,20]. A recent analysis of a single institutional experience with BCS demonstrated that LRR rates are low for patients treated either with initial surgery (6% at 10 years) or NAC (10% at 10 years), and the initial treatment modality had no significant impact on LRR-free survival rates after adjusting for the clinical stage at presentation [21].
No uniform definition of surgical margin status has been established. A general consensus was reached by a series of published studies indicating that a surgical margin is positive if cancer cells are immediately at the edge of the resection on an inked histology section, according to investigators from the National Surgical Adjuvant Breast and Bowel Project [22]. In contrast, 1 mm is currently designated as the minimal distance for a positive surgical margin according to the European Institute of Oncology [23]. A huge population meta-analysis (LRR in 1,506 of 28,162) reported that a negative margin reduces LRR in patients with early-stage breast cancer and BCS. However, increasing the distance used to define a negative margin did not result in a significantly reduced LR [7].
Acceptable safety margins in patients with BCS after NAC are even more controversial [6], as they differ from those for patients undergoing conventional BCS. Large tumors on presentation may not be uniformly destroyed by chemotherapy; hence, a negative-resection margin is not guaranteed. Furthermore, determining original tumor size is difficult, particularly when a major response to NAC is obtained [9,10]. Nevertheless, most breast oncologic surgeons aim to obtain negative-resection margins for local control and tend to excise large volumes of tissue during BCS after NAC [15].
Reoperation after BCS because of positive margins is common and re-excision rates vary widely, but are generally 15% to 40% [24,25]. Two main factors must be considered to reduce the reoperation rate following detection of positive margins. First, it is essential to determine the extent of resection, particularly in patients who have a major response to NAC and MRI is an established tool to evaluate this response. Despite the superior accuracy of MRI compared with other modalities (breast mammography and ultrasound), residual tumor extent can be over- or underestimated [26]. Several reports have suggested that the diagnostic reliability of conventional MRI may vary according to cancer subtype; lower accuracy was associated with the luminal subtype rather than the triple-negative and HER2-positive phenotypes [27,28]. In our study, HR positivity was a preoperative predisposing factor for positive frozen- or permanent-section margins. Second, evaluation of intraoperative strategies that could reduce positive margins would be useful, including localization by intraoperative ultrasound, radio-guided localization, specimen radiographs, or intraoperative margin assessment such as gross evaluation only or microscopic margin evaluation including imprint cytology or frozen-section analysis. In our study, we performed appropriate localization, specimen radiography, and frozen-section biopsies, and found that the latter had a high specificity (98.5%). We found a low positive-margin rate on permanent sections (4/151, 2.7%).
The median follow-up after BCS was 49.4 months (range, 2.3–110.4 months). Only one patient developed local recurrence, four had regional recurrence, and 21 had distant recurrence. Only one local recurrence was detected by imaging follow-up 37.5 months after BCS. We surmise that this relatively low LRR was due to the use of appropriate adjuvant therapies such as radiation, hormonal, and HER2-targeted therapies. In our study, almost all patients underwent radiation therapy, and the primary tumor bed was boosted in 83.1% (123/148). Some reports indicate that radiation therapy offers improved local control. Huang et al. [29] found that comprehensive radiation benefits both local control and survival in patients presenting with clinical T4 tumors or stage III–IV disease, in addition to those with four or more positive nodes in the NAC setting. Bartelink et al. [30] reported that boost radiation of the tumor bed led to improved local control, but no difference in survival after a median follow-up period of 10.8 years.
Our study had several limitations. First, we used a number of NAC regimens due to differences in the NAC response; there were eight NAC regimens but four inclusive groups were defined, largely because some of the NAC regimen groups had few patients. Nevertheless, we focused mainly on negative margins in patients who underwent BCS after NAC and evaluated their clinical response. Second, a positive frozen-section margin was assumed to indicate a positive-margin status. We had very few positive permanent-section margins (4/151, 2.7%) because we always performed a frozen-section biopsy, and additional resection was performed to obtain negative frozen-section margins intraoperatively. If we did not perform frozen-section biopsies, positive permanent-section margins would have been considered. Strikingly, 49% of the audience at the 15th Annual Meeting of the American Society of Breast Surgeons were not using any intraoperative margin-assessment method [8]. We consider using a method such as frozen-section biopsies to be extremely important. Third, this was a retrospective study and the sample size was not very large. However, our median follow-up period was 49.4 months, which is longer than that of previous studies.
Despite these limitations, our results have important clinical implications. First, HR positivity and clinical multifocality after NAC were predisposing factors for positive margins in patients with BCS after NAC. Second, despite the short-term follow up, there were only four cases of LRR, suggesting that negative margins at the margin cut and appropriate adjuvant therapy are necessary for patients undergoing BCS after NAC.
In conclusion, our retrospective study revealed that HR positivity and clinical multifocality were significantly associated with positive margins. We encourage surgeons to obtain safe resection margins to avoid positive margins in patients with these clinicopathological factors.
Notes
The authors declare that they have no competing interests.