Case Report of Desmoid Fibromatosis as a Rare Disease Entity of the Breast
Article information
Abstract
Desmoid fibromatosis (DF) of the breast is a rare condition. Owing to its rarity and mimicry of carcinoma, the diagnosis and optimal treatment of DF are challenging tasks. A 41-year-old woman with no history of augmentation or other breast procedures for breast cancer was examined. The patient had a palpable mass in the left breast identified as a spiculated hypoechoic mass using ultrasonography. Core needle biopsy revealed spindle cells without mitotic figures. The mass was excised and a histopathological examination confirmed the diagnosis of DF. The patient had no history of any related factors. Imaging studies did not reveal specific features that could distinguish this tumor from other commonly presenting carcinomas. Therefore, pathological confirmation is of paramount importance for the diagnosis of DF. Further experience with more cases will help to understand DF better and develop a multidisciplinary approach to its clinical management.
INTRODUCTION
The World Health Organization defined desmoid fibromatosis (DF) as a clonal fibroblastic proliferation derived from deep soft tissue capable of local infiltration [1]. DF is a rare disease that specifically affects the breast. DF accounts for only 0.2% of all breast neoplasms, and its etiology has not been established [2]. Through imaging studies, DF has been revealed to mimic carcinoma in appearance [3,4]. Owing to its rarity and mimicry of carcinoma, the diagnosis and optimal treatment of DF are challenging tasks [5]. Histopathological analysis, which reveals the proliferation of spindle cells in collagen deposits, is paramount for DF diagnosis [6,7]. Herein, we describe an uncommon DF at our institution and review the literature relative to our case.
CASE REPORT
Patient information
A 41-year-old woman visited the local clinic with a palpable mass in the left breast. Ultrasound-guided biopsy was performed for a hypoechoic lesion classified as Breast Imaging Reporting and Data System (BI-RADS) category 4. The pathological examination revealed bland spindle cells without mitotic figures. The patient was referred to our institution for further treatment.
The patient was gravida 1 and had a history of lactation but had no medical history, including trauma, cancer, or breast augmentation. She had previously used oral contraceptive pills; however, the type and duration were unclear. Physical examination revealed a palpable mass in the left lower quadrant that had been present for 4 weeks prior to the outpatient visit. No signs of inflammation or skin retraction associated with lesions were found.
The study protocol was approved by the Institutional Review Board (IRB) of our institute (IRB No. NCC 2022-0237). This study was conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was waived due to the retrospective nature of the study.
Diagnosis
An imaging workup of the whole breast and axilla was performed before surgery. Mammography revealed no remarkable findings, except for asymmetry in the contralateral breast (Figure 1). Ultrasound examination revealed an irregular spiculated hypoechoic mass with increased vascularity, measuring 1 cm in its largest diameter in the left lower outer quadrant, located in the pre-mammary layer of the breast (BI-RADS category 4B), and no axillary lesions (Figure 2). A repeat biopsy was performed at the patient’s request, returning the same pathological findings as the previous biopsy performed at another institution. Another suspicious lesion was classified as BI-RADS category 4A in the contralateral breast; however, based on a core needle biopsy, the lesion was identified as a fibroadenoma.
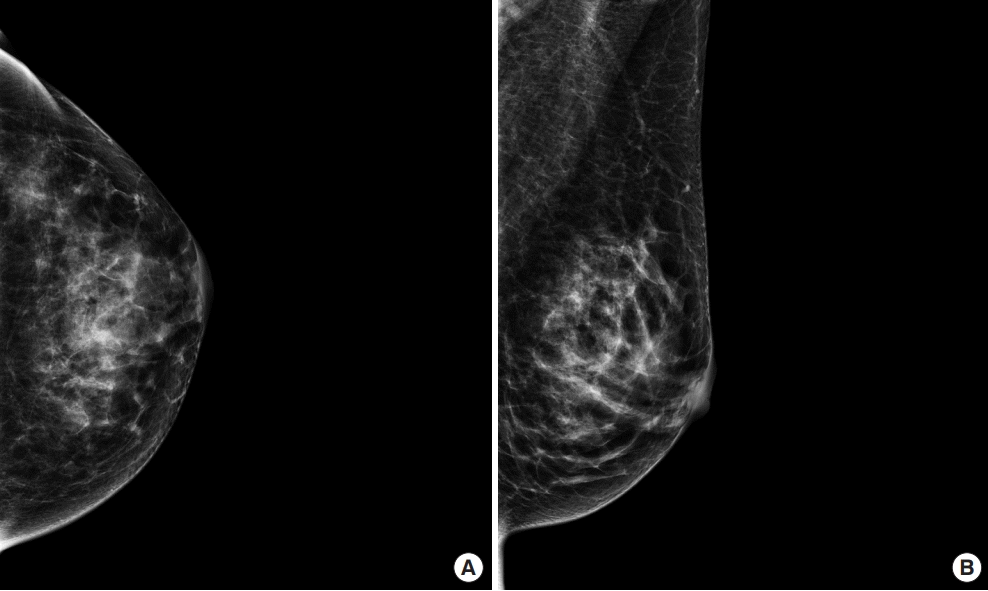
Mammography (A) Craniocaudal and (B) mediolateral oblique view. Mammography revealed no remarkable findings, except for asymmetry in the contralateral breast.
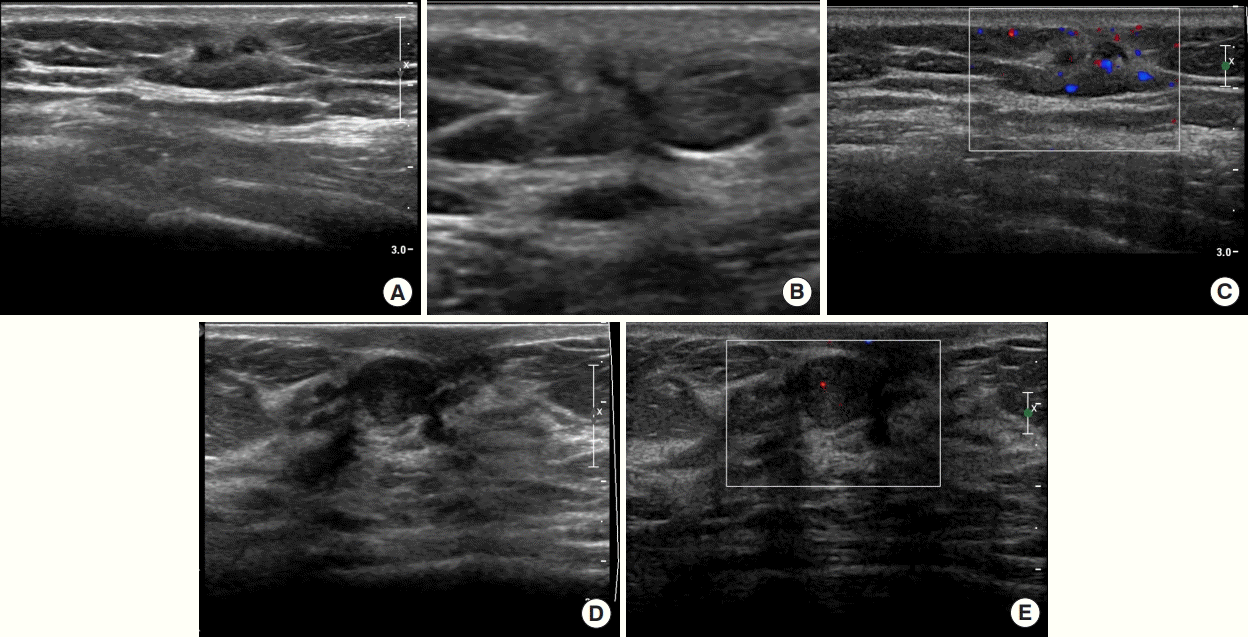
In desmoid fibromatosis, (A, B) B-mode ultrasonography (US) images (transverse and longitudinal views) show a 1 cm irregular hypoechoic mass with spiculated margin in the left lower-outer quadrant. This lesion was classified as Breast Imaging Reporting and Data System category 4B. (C) Color Doppler US image shows high vascularity. In fibroadenoma, (D) B-mode US image shows a 1 cm oval hypoechoic mass with circumscribed margin in the right subareolar region. This lesion was classified as Breast Imaging Reporting and Data System category 4A. (E) Color Doppler US image shows low vascularity.
Surgery and pathology
The patient underwent mass excision of the lesion in the left breast with diagnostic intent. The histopathologic report revealed a 0.9 × 0.8 × 0.5 cm spindle cell lesion with no mitosis, no necrosis, infiltrative tumor border, and clear resection margins. Some areas also harbored dense eosinophilic stromal collagen. Microscopically, the tumor cells were characterized by long fascicles of bland myofibroblasts with pale eosinophilic cytoplasm. Nuclear staining was positive for smooth muscle actin and negative for CD34, S-100, and pan-cytokeratin. Some tumor cells exhibited nuclear beta-catenin expression. The immunophenotypic appearance was compatible with that of DF (Figures 3-5).
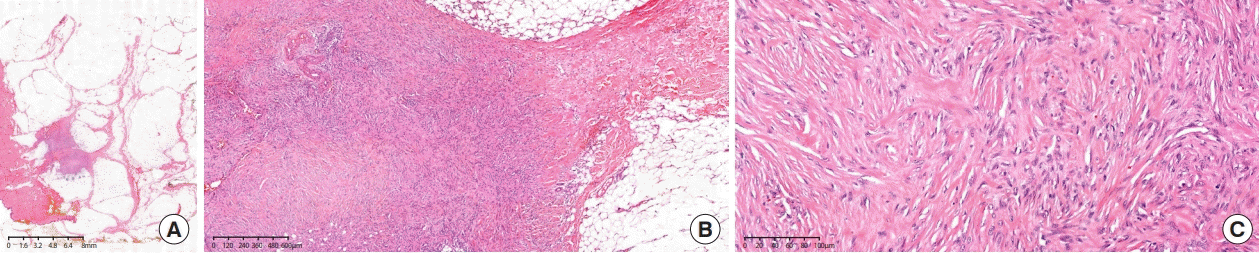
Hematoxylin and eosin staining. (A) At 1×, low-power examination reveals a lesion infiltrating the surrounding soft tissue with lymphoid aggregates. (B) At 40× (objective field), the tumor has areas with keloid-type collagen deposition. (C) At 200×, low-power examination reveals bland myofibroblasts without cytological atypia.
Additional treatment
The patient underwent regular follow-up with ultrasound 6 months after surgery without any additional treatment. However, no specific findings were obtained. Mammography and breast ultrasonography were scheduled for breast examination after 1 year.
DISCUSSION
Most DF cases manifest in the abdomen; however, extra-abdominal desmoid tumors account for approximately 4% of all desmoid tumors [1]. DF in the breast is rare, accounting for 0.2% of all breast neoplasms. The etiology of this condition has not been clarified. Hormonal and genetic factors are reportedly associated with DF genesis [8-10]. Trauma and surgical procedures, including augmentation with silicone implants, have also been reported to affect DF [10]. Bouab et al. [11] described a similar case of DF after breast reduction surgery. In the present case, the patient diagnosed with infiltrating ductal carcinoma underwent a mastectomy, axillary lymph node dissection of the right breast, and contralateral breast reduction. One year after surgery, the 2 cm nodules found in the left breast were confirmed to be DF. Ebrahim et al. [12] reported the patient demographics and clinical presentations of six fibromatosis cases of the breast over 10 years. One of the patients had a history of breast surgery. Previously, Hammood et al. [9] reported a case of DF in the breast and mentioned no identified risk factors except for oral contraceptive use. Similarly, no correlation was found with factors besides oral contraceptive use in the present case. With regard to hormonal factors, previous reports and the present case suggest that oral contraceptive use could be a risk factor.
DF in the breast appears similar to carcinoma on physical examination and imaging studies, making it challenging for clinicians to differentiate DF from malignancy [3]. Lorenzen et al. [4] reported 15 cases over a period of 10 years that were identified through a digital archive search. The clinical presentation and imaging findings were not specific to DF. Most patients had a palpable mass, and some had skin retractions. Some studies have suggested that preoperative magnetic resonance imaging could be helpful in assessing the total extent and chest wall invasion, but not differential diagnosis [12,13].
DF is diagnosed via core needle biopsy [7]. This diagnosis can be difficult because spindle cells can indicate various diseases, including nodular fasciitis, phyllodes tumor, and fibrosarcoma. A diagnostic excisional biopsy provides a greater chance of a confirmatory diagnosis. Microscopic examination revealed spindle cell proliferation and collagen deposition. The mitotic rate is low. The margin is infiltrative, extending into the surrounding tissues [12]. Additional information from immunohistochemistry, such as the expression of smooth muscle actin without the expression of cytokeratin, CD34, or S-100, plays an adjunctive role in the diagnosis.
No consensus has been reached regarding the treatment of DF of the breast. In the past, wide excision was performed for most cases of desmoid tumors. According to the European Consensus, it is not clear whether en bloc surgery should be the method of choice, as a progression-free disease course and spontaneous regression are documented in up to 88% of cases. No significant benefit was observed in active treatments, such as antihormone therapy, chemotherapy, and radiation therapy, compared to patients being observed [6]. Owing to the rarity of cases in the breast, insufficient information is available to compare such cases. A large prospective nationwide study on desmoid tumors revealed primary location as a prognostic factor for event-free survival (EFS) [1]. In this study, DFs were categorized as having unfavorable (such as the chest wall, head and neck, and upper limbs) or favorable locations (such as the abdominal wall, intra-abdominal, breast, digestive viscera, or lower limb). In cases with unfavorable locations, the 2-year EFS was 51.8% with the wait-and-see approach compared to 25.4% with surgery. However, surgery and active surveillance resulted in similar oncological outcomes in patients with favorable tumor locations (EFS: 62.8 vs. 69.6%). DF tends to recur frequently in up to 27% of cases. Two-year EFS was 32% after open biopsy compared to 57% after a core needle biopsy, suggesting that a proliferative wound healing process could be associated with recurrence. However, negative margins cannot exclude the possibility of recurrence.
As only few case reports are available on this disease entity, DF remains poorly understood. Further experience with more cases will help to better understand DF and develop a multidisciplinary approach to its clinical management.
Notes
The authors declare that they have no competing interests.

