INTRODUCTION
Breast metastases from extra-mammary malignancies are rare [1], and in particular, breast metastases from gastric cancer are even rarer. The common symptoms in all reported cases of breast metastases, including from gastric cancer, are a non-tender palpable mass, diffuse edema of the skin, or erythema. However the radiologic features of secondary breast malignancies tend to indicate a benign tumor [2,3]. Therefore even though there are no typical malicious imaging findings of palpable breast masses, clinicians should consider the possibility of breast metastases and perform a histological examination in patients with a history of extra-mammary malignancies.
CASE REPORT
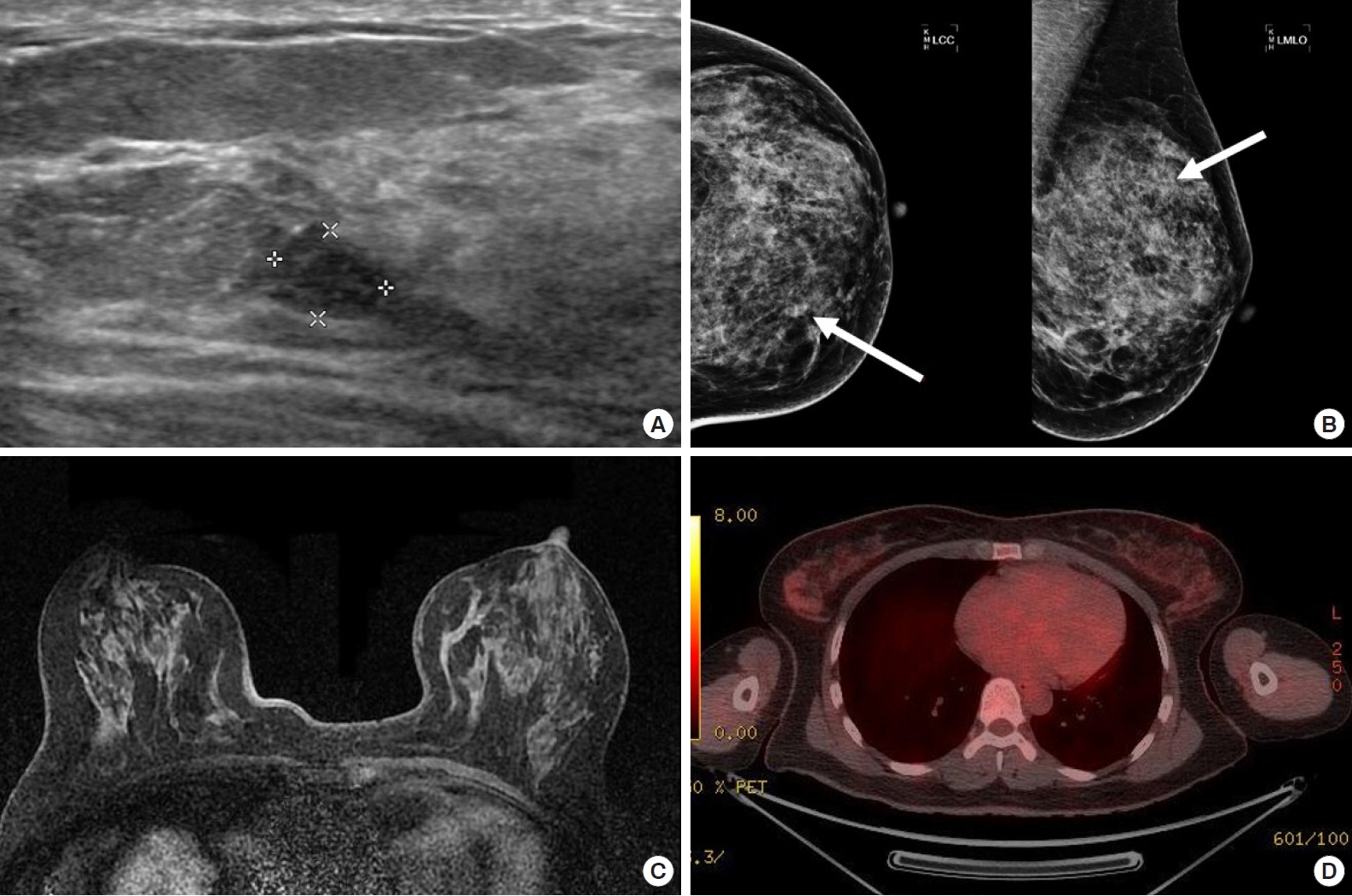
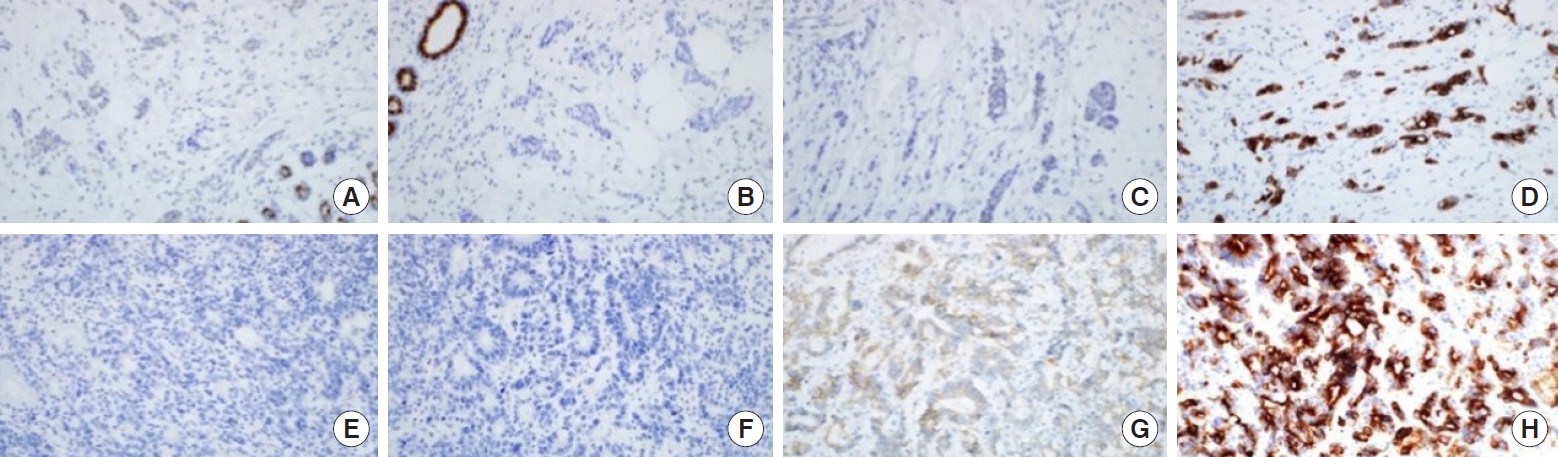
A 41-year-old female asymptomatic patient was on annual follow-up since February 2014 for a non-palpable right breast nodule that was detected on a screening mammography. One year after the first visit, a hypoechoic mass was found in the 10 o’clock position at a 4cm distance from the nipple. The ultrasonographic feature of the mass seemed benign because it was well-circumscribed and the architectural structure was preserved. In mammography, a new isodense mass was found in left breast upper-inner quadrant. A ultrasound-guided core needle biopsy of the mass was performed and it was later confirmed as an malignancy and positron emission tomography/computed tomography (PET/CT) and breast magnetic resonance imaging (MRI) were performed for initial staging. On PET/CT, there was no evidence of hypermetabolism around the proven malignant mass. Instead, a new mass-like consolidation was observed at the 5 o’clock position of the left breast. In addition, the malignant mass in the left breast was not noted on MRI (Figure 1). The breast surgeon planned to perform a left mastectomy with ipsilateral sentinel lymph node biopsy. Intraoperative frozen biopsy for the left axillary sentinel lymph nodes was positive for malignancy; thus, a left modified radical mastectomy was performed in April 2015. Multifocal metastatic adenocarcinomas were detected in the mastectomy specimen, and the largest one measured 4.3× 2.2 cm. Eighteen out of the 19 dissected axillary lymph nodes were positive for malignancy. On immunohistochemistry, the tumor cells were negative for estrogen receptor, progesterone receptor, and HER-2, but positive for p53, CK7, and E-cadherin (Figure 2). The patient previously underwent surgery for advanced gastric cancer in October 2013. The stage of gastric cancer was IIa (pT1bN2M0). The cell type was tubular adenocarcinoma, poorly differentiated. She completed 6 cycles of adjuvant chemotherapy with the capecitabine plus oxaliplatin (XELOX) regimen approximately 1 year before the breast surgery. Multiple metastatic skin masses were found 2 months after the modified radical mastectomy of the left breast. She received palliative chemotherapy with 6 cycle of fluorouracil plus irinotecan (FOLFIRI) and 3 cycle of Paclitaxel/carboplatin. She died in August 3, 2016. Progression free survival was 14 months. The characteristics of the case is summarized in Table 1. This case study was approved by our Institutional Review Board, and the requirement for written consent was waived (IRB No. 2020-10-087).
DISCUSSION
The first case of breast metastasis from gastric cancer was reported by Reitmann et al. in 1908, but since then, only 45 cases (including ours) have been reported. Moreover, only one patient was a male [4-6]. The most common symptom among all reported cases of breast metastases from any malignancy is a non-tender palpable mass [3]. Similarly, most cases of breast metastases from gastric cancer initially presented as a palpable lesion of the breast [2,4-11]. Except for the 12 cases with inconclusive symptoms, 22 of the 33 reported cases (66.6%), including our case, had palpable lesions. Meanwhile, the remaining 33.4% have similar symptoms of inflammatory breast cancer such as diffuse edema of the skin or erythema.
The median age of patients with breast metastases from gastric cancer is relatively younger than that of patients with primary breast cancer [5]. Given the relatively young age of premenopausal patients, it can be inferred that hormonal factors may influence the pathogenesis of the breast metastasis [12]. Estrogen affects tumor metastasis in two ways: (1) direct effect on the tumor cell cycle and (2) modulation of the peritumoral microenvironment. While it is inconclusive and we do not know the exact mechanisms, substantial evidence supports that an elevation of the level of circulating bioactive estrogen can directly affect carcinogenesis and tumor cell growth in patients with hormone receptor-positive cancer. However, the histological type of our case is signet ring cell carcinoma, and the estrogen receptor is not expressed in this case; therefore, there is no evidence for a direct effect of estrogen in breast metastasis from gastric cancer in this case.
In the previously reported 42 cases of metastatic breast cancer from gastric cancer, excluding three cases of no site information, 54.7% (23/42) were located in the left side of the breast. As such, some authors suggested retrograde lymphatic spread hypothesis, i.e., cancer cells spread from regional lymph nodes to the breast via specific lymphatic pathways that extend from the extra-mammary organs to the breast such as the left supraclavicular lymph node [2]. However breast metastases from extra-mammary malignancies and from gastric cancer develop in the both side of breast. Regional lymph node metastases in left breast metastasis from gastric cancer is rare, accounting for only 26.0% (6/23) of all reported cases; moreover, lymph node involvement is even rarer in metastatic breast cancer from extra-mammary malignancies [13].
It is challenging for radiologists to distinguish between primary breast cancer and breast metastasis. The ultrasonographic features of breast metastases from extra-mammary malignancies vary considerably. Breast metastases from extra-mammary malignancies are classified into four categories, namely, typical hematogenous, typical lymphatic, atypical mass lesion, and atypical non-mass lesion, and described the ultrasonographic features of each category [2,3]. The features of each group are summarized in Table 2. In the 45 reported cases of breast metastases from gastric cancer, including ours, 29 cases have no records of ultrasonographic features. Only 10 cases were described as indistinct hypoechoic masses (n = 5), diffuse hypoechoic non-mass lesions (n =2), hypoechoic mass (n =2, no record about the margin), well-circumscribed hypoechoic mass (n =1, our case), and skin thickening (n =3). There are no specific ultrasonographic findings for breast metastases from gastric cancer, and similar findings were obtained from other imaging modalities such as mammography, MRI, or PET/CT. In our female patient, PET/CT and MRI findings did not coincide with the ultrasonographic findings.
Diagnosing breast metastasis from gastric cancer is difficult due to the similar pathology between the primary breast cancer and the primary gastric cancer. The unusual type of invasive lobular carcinoma have signet ring cells and 20%–40% of gastric cancer stain positively for the estrogen receptor on immunohistochemistry. Although most reports discussed the absence of in-situ components and the periductal location of tumor cell as hallmarks of metastatic cases, it does not accurately exclude primary breast cancer. Most cases of breast metastasis from gastric cancer are signet ring cell carcinoma, and it is hard to distinguish the metastatic mammary signet ring cell carcinoma from the primary gastric cancer on hematoxylin and eosin staining. There is no single conclusive method for diagnosing breast metastasis; thus, it is useful to compare the biopsy slide of the breast lesion with that of the primary site, and an integrative decision should be made with clinical, radiological, and pathological data [14]. In our case, the preoperative diagnosis of core needle biopsy specimen was intraductal carcinoma, which was misdiagnosed because the pathologist had no clinical information and primary breast cancer cells also have both glandular or ductal differentiation.
Similar to conventional markers for primary breast cancer, several tumor markers could assist the pathologic diagnosis of breast metastases from gastric cancer. Breast metastasis tumors usually stain positively for CEA, CK7, CK20, or E-cadherin on immunohistochemistry, similar to gastric signet ring cell carcinoma. The tumor makers specific to gastric cancers such as HIK1083 or MUC families could also be used for diagnosis [5]. Our female patient stained positively for CK7 and E-cadherin, and the mastectomy specimen had extensive tumor emboli in the lymphovascular spaces without in situ components and was similar to the primary gastric cancer slide on H&E staining. As such, we concluded that the breast lesion was a metastatic lesion.
To select the appropriate treatment modality for the patients with metastatic breast cancer from gastric cancer, it is necessary to understand that these patients have poor prognosis. Most cases of metastatic breast cancer from gastric cancer are advanced. Despite the absence of extra-mammary metastases, there is a high potential for systemic dissemination of the primary cancer. The overall 1-year survival rate is less than 20% [1,7]. Thus, radical operation should not be performed except for the relief of symptoms such as continuous nipple discharge or severe pain. Iesato et al. [4] reported a case of combination chemotherapy of S-1 and cisplatin that was tolerable and effective for the reduction of the breast mass. However, in most cases, chemotherapy was ineffective because of disseminated metastatic disease and poor general condition. Meanwhile, hormonal therapy is rarely performed due to an absence of hormone receptor in immunohistochemical stains.
Breast metastases from gastric cancer might be a heterogeneous disease entity based on the clinical, radiological, and histopathological inconsistencies. Because of its rarity, a multicenter study is necessary to obtain more data and to increase the statistical power of findings. Currently, there is no standard and accurate diagnostic modality for breast metastases, and patients are diagnosed based on the clinician’s suspicion and combination of objective findings.