INTRODUCTION
Breast cancer has several prognostic factors, such as tumor size, lymph node metastasis, and distant metastasis that determine the treatment strategy. Moreover, the evaluation of the hormone and human epidermal growth factor receptor 2 (HER2) status are essential for determining the appropriate treatment [1].
Since the advent of routine mammography screening, the breast cancer tumor size at initial diagnosis has markedly decreased due to earlier detection [2]. However, as the detection limits of palpation are approximately equal to 1 cm or greater, the incidence of small (less than 1 cm) invasive breast cancer without axillary lymph node involvement has been increasing [3,4].
Node-negative T1a breast cancer has been shown to have a relatively good prognosis, regardless of the hormone receptor status. Five-year disease-free survival (DFS) of T1aN0M0 breast cancer was over 95% [5]. The 2016 National Comprehensive Cancer Network guidelines (version 2), after surgery for T1aN0 breast cancer, do not recommend adjuvant treatment in hormone receptor-negative breast cancer patients, but state that adjuvant endocrine therapy may be considered for hormone receptor-positive of the same grade patients. The 2016 American Society of Clinical Oncology guidelines state that patients with T1aN0 breast cancer may not benefit from adjuvant chemotherapy. Nevertheless, in patients with hormone receptor-negative T1aN0 breast cancer, treatment with adjuvant chemotherapy remains controversial. Thus, no consensus has been reached regarding the extent of benefit from adjuvant systemic therapy, and there are no clear related guidelines for T1aN0 patients [1].
The fluoropyrimidine 5-fluorouracil (5-FU) was synthesized by Heidelberger et al. and Duschinsky et al. in 1956. This drug has played a central role in chemotherapy for solid tumors, and remains an important component of combination chemotherapy regimens for breast cancer [6]. An oral 5-FU derivative, doxifluridine (Didox; Shin Poong Pharm. Co., Ltd., Seoul, Korea), which is a prodrug of 5-FU and a metabolite of capecitabine (Xeloda; F. Hoffmann-La Roche, Basel, Switzerland), is an option for adjuvant chemotherapy [6,7]. It has lower adverse effects than intravenous (IV) 5-FU, including toxicity to the bone marrow, immune-suppression, and alopecia [6,8]. Although it has a side effect profile including mild diarrhea, nausea, and vomiting, in general it possesses a high tolerance and favorable safety [8]. The findings of a phase II study of doxifluridine in patients with advanced/recurrent breast cancer indicated a 35.9% response rate with lower toxicity when compared to IV 5-FU and tegafur [9]. Furthermore, oral 5-FU was as effective as cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) chemotherapy for node-negative, high-risk breast cancer patients [10]. The CMF versus 5-FU in breast cancer trial indicated that oral 5-FU was noninferior to CMF in patients with stage I to IIIa breast cancer and 1 to 9 metastatic axillary nodes [6].
In the present study, we aimed to assess the effect of doxifluridine for T1aN0 hormone receptor-negative breast cancer patients.
METHODS
Data were obtained from the Asan Medical Center (AMC) database. The AMC database is a prospectively maintained, web-based system that includes information on all patients who have undergone operations for breast cancer at the AMC in Korea since 1989. To date, more than 20,000 patients with breast cancer have been registered, providing detailed clinicopathological information related to their breast cancer. Eight surgical oncologists specializing in breast cancer reviewed all available clinical pathology and survival data for this study. The recurrence and mortality of all patients was evaluated; the database includes detailed information on patient recurrence, breast cancer-specific survival (CSS), and overall survival.
Cohort
We retrospectively identified pT1N0 and hormone receptor-negative breast cancer patients who underwent surgery between 1993 and 2008 at AMC. Tumor staging was based on the TNM system (American Joint Committee on Cancer seventh version). Eligibility criteria included complete surgical resection, histological diagnosis of invasive ductal or lobular carcinoma (less than or equal to 0.5 cm) involving micro-invasion, no lymph node metastasis (T1aN0), and hormone receptor-negative status. All patients received locoregional treatment such as modified radical mastectomy, breast-conserving surgery, axillary lymph node dissection or sentinel node biopsy, and postoperative radiation therapy depending on the state of the disease. Patients receiving adjuvant chemotherapy (CMF, or doxorubicin and cyclophosphamide [AC]) and/or endocrine therapy were excluded. Patients who received anti-HER2 targeted therapy were also excluded. Follow-up was performed with physical examination, chest radiography, and mammography annually. Positron emission tomography-computed tomography, ultrasonography, chest computed tomography, and abdomen and pelvis computed tomography were performed, if required.
Patients were divided into two cohorts based on whether adjuvant doxifluridine medication was administered. In cohort I (doxifluridine group), doxifluridine (400 mg) was administered orally twice a day for 6 months, whereas in cohort II (surgery only group), only surgery was performed.
Pathology data were obtained by reviewing pathology reports tumor size, histologic grade, and nuclear grade. The immunohistochemistry (IHC) data for the HER2 status were also collected. Cases with HER2 expression level 3+ on IHC staining were considered positive, whereas the remaining cases were considered negative. As patients in the present study underwent surgery before 2008, minimal fluorescent in situ hybridization and silver in situ hybridization data were available for HER2 2+ tumor on IHC.
Endpoints
The endpoint of this study was the comparison of the DFS between cohort I and II. DFS was defined as the duration between surgery and the final follow-up without recurrence or the first examination with recurrence on biopsy or imaging. The secondary outcomes included CSS. CSS was defined as the time from surgery to death due to breast cancer. The detection of contralateral invasive breast cancer was not considered recurrence.
RESULTS
Of 299 patient records meeting the clinical inclusions criteria for the study, 50 patients did not meet the inclusion criteria due to receiving adjuvant chemotherapy; hence, 249 patients were finally assessed. The number of patients in the doxifluridine and surgery only groups was 198 and 51, respectively. The age and tumor size were similar between the doxifluridine group and surgery only group (Table 1). The number of HER2-positive patients in the doxifluridine group was 147 (74.2%), whereas that in the surgery only group was 31 (60.7%). Micro-invasive tumors were considered to have a size of 0.1 cm.
Survival
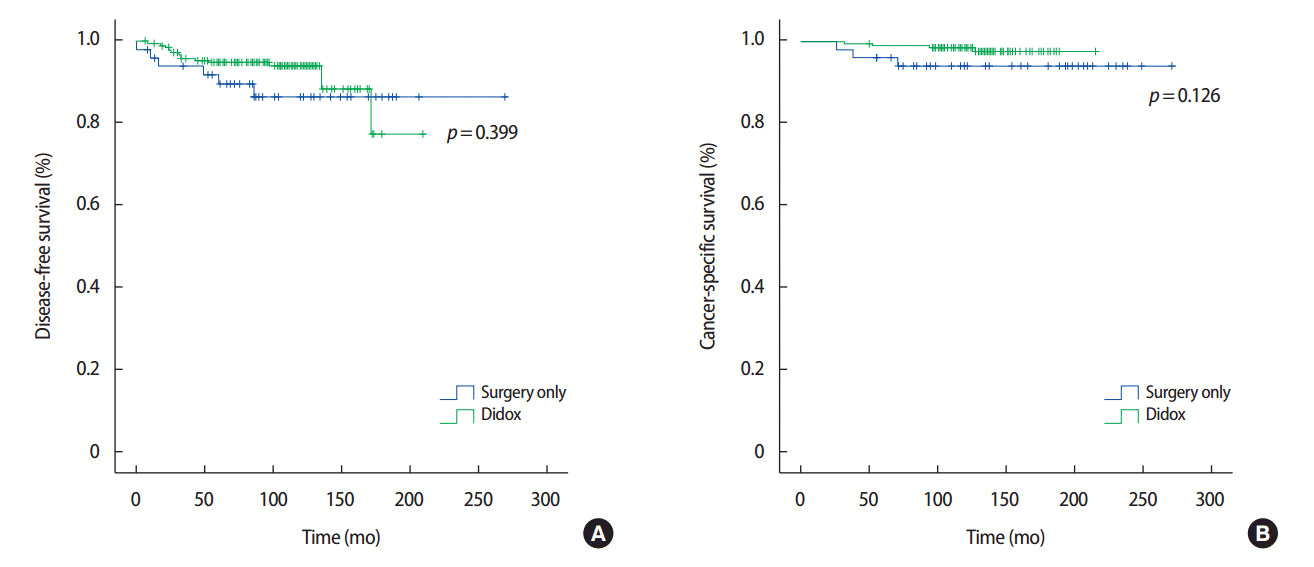
Figure 1 displays the DFS and CSS of the cohorts. The DFS and CSS did not significantly differ between the groups (p=0.399 and p= 0.126, respectively). The recurrence rate was 6.1% (12/198) and 11.8% (6/51) in the doxifluridine and surgery-only groups, respectively. Recurrence was classified as local, regional, and distant recurrence (Table 2). Local, regional, and distant recurrence was observed in 2.5% (5/198), 2.5% (5/198), and 1.0% (2/198) cases in the doxifluridine group and 5.9% (3/51), 3.9% (2/51), and 2.0% (1/51) cases in the surgery-only group, respectively. There were two cases of distant recurrence in the doxifluridine group, including a case of lung and bone metastasis 13 years after surgery, and a case of lung metastasis at 4 years after surgery. There was a case of distant recurrence in the surgery only group, wherein contralateral axillary lymph node recurrence was noted 4 years after surgery.
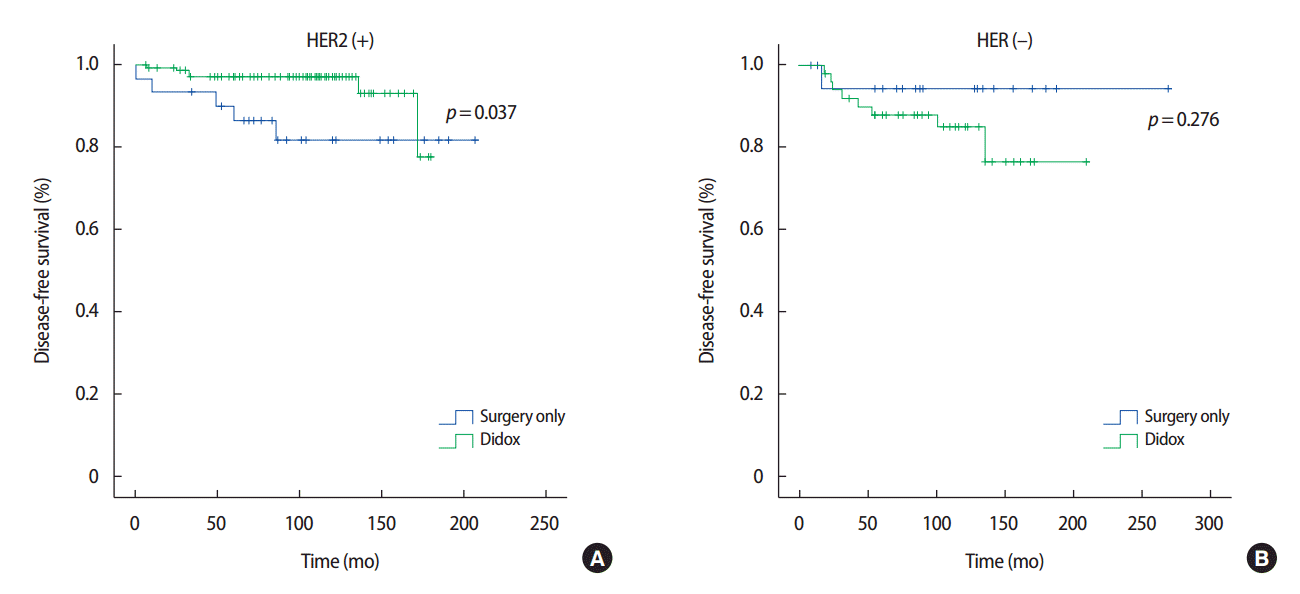
Figure 2 illustrates the DFS stratified by the HER2 status. Doxifluridine exhibits a significant beneficial effect on the DFS in T1aN0 and HER2-positive breast cancer patients (p=0.037). In contrast, the DFS of T1aN0 and HER2-negative breast cancer patients did not significantly differ according to doxifluridine treatment (p=0.276).
DISCUSSION
The prognosis is known to be good in T1aN0 breast cancer, but can be poor in specific subtypes, such as hormone receptor-negative and HER2-positive diseases. In fact, among T1abN0 hormone receptor-negative breast cancer patients, the prognosis of HER2-positive breast cancer was worse than HER2-negative breast cancer. HER2-positive breast cancer patients had a recurrence rate that was three times higher than that of HER2-negative breast cancer patients (hazard ratio, 3.13; 95% confidence interval, 1.23–7.92; p=0.016) [11].
Oral 5-FU derivatives, including tegafur-uracil (UFT), tegafur-gimeracil-oteracil potassium (S-1), doxifluridine, and capecitabine, are used for chemotherapy in breast cancer [6]. Since its introduction in the 1990s, oral 5-FU has been widely used as postoperative chemotherapy for breast cancer, due to the convenience of administration and the low incidence of serious adverse events [6]. The Adjuvant Chemoendocrine Therapy for Breast Cancer trial confirmed the effectiveness of UFT as postoperative chemotherapy for breast cancer, and this drug had since been widely used in general clinical practice [12]. Some studies of S-1 in patients with advanced or recurrent breast cancer have been reported [6]. The response rates were found to be 40.7% and 42.0% in patients who received S-1 as first- and second-line treatment, respectively; in contrast, patients who did not respond to anthracycline or taxanes exhibited a response rate of 21.8%. Thus, S1 has high antitumor activity [13,14].
The present study also showed a survival benefit of HER2-positive hormone receptor-negative small breast cancer. The risk of recurrence in T1aN0 hormone receptor-negative breast cancer remains unclear, and the benefit of any adjuvant therapy remains uncertain. In fact, trastuzumab-based adjuvant therapy has been found to be useful in T1bN0 and HER2-positive breast cancer patients [15]. However, some retrospective studies indicated poor prognoses in a certain subset of small cancers. In particular, Gonzalez-Angulo et al. [16] assessed 965 patients with small (≤1cm) node-negative breast cancers who did not receive adjuvant chemotherapy or trastuzumab, and found a higher risk of recurrence in HER2-positive and triple-negative subtypes. Moreover, Curigliano et al. [17] examined 150 patients with pT1abN0 and HER2-positive breast cancer, and found a low risk of recurrence at 5 years; however, among patients with hormone receptor-positive cancer, HER2 overexpression was associated with a poorer DFS.
Several previous studies have described the usefulness of doxifluridine in early breast cancer. Doxifluridine monotherapy for 6 months did not yield any survival benefit as compared to surgery alone in early breast cancer [8]. However, our present study series included patients with node-negative and -positive breast cancer, and hence, in this population, doxifluridine alone may not be a sufficient adjuvant treatment; therefore, combined treatment with other standard chemotherapy would be more effective. Moreover, didox was not as therapeutically useful as the standard treatment in older women with early breast cancer [18]. In the CREATE X-trial [19], which compared the addition of capecitabine to standard neoadjuvant chemotherapy, it was found that adjuvant capecitabine improves outcomes in women with HER2-negative breast cancer with residual invasive disease after neoadjuvant chemotherapy. Moreover, the balance of benefits and toxicity favors the use of capecitabine in postneoadjuvant chemotherapy conditions [19]. The antitumor activity in breast cancer and lower toxicity of 5-FU make it an ideal candidate for the treatment of specific types of breast cancer, regardless of whether it is combined with chemotherapy.
We here assessed the effectiveness of doxifluridine in hormone receptor-negative, HER2-positive T1aN0 breast cancer. In the HER2- positive breast cancer patients, trastuzumab-based adjuvant therapy exhibited a benefit, even if the tumor size was very small and it was node negative [15,20]. However, in some countries, trastuzumab cannot be prescribed to HER2-positive breast cancer patients due to cost or reimbursement issues. Hence, doxifluridine may be useful for patients in those countries.
This study was retrospective in nature and hence had some inherent limitations. Due to the relatively small study size, there was a possibility of selection bias. However, both the cohorts had a similar age and tumor size. We carefully examined patients with T1aN0 hormone receptor-negative breast cancer who did not receive clear adjuvant treatment strategies. Several studies have reported the prognosis and treatment of T1a,bN0 breast cancer, but data on T1aN0 hormone receptor-negative breast cancer are rare. Moreover, based on our current findings, HER2-positive hormone receptor-negative T1aN0 breast cancer patients may be treated using doxifluridine, particularly in countries where trastuzumab cannot be prescribed. Nevertheless, a randomized controlled trial to assess the effect of doxifluridine in T1aN0 hormone receptor-negative HER2-positive breast cancer patients would be considered necessary.
In conclusion, doxifluridine did not benefit DFS outcomes in T1aN0 hormone receptor-negative breast cancer patients. However, in HER2-positive and T1aN0 hormone receptor-negative breast cancer patients, doxifluridine exhibits a survival benefit.